Hydrogel-Encapsulated MSC Exosomes: A Sustained-Release Strategy for Advanced Wound Healing
Chronic wounds represent a significant clinical challenge, driving the need for innovative regenerative therapies.
Hydrogel-Encapsulated MSC Exosomes: A Sustained-Release Strategy for Advanced Wound Healing
Abstract
Chronic wounds represent a significant clinical challenge, driving the need for innovative regenerative therapies. This article explores the combination of mesenchymal stem cell (MSC)-derived exosomes—nanoscale vesicles with potent regenerative, anti-inflammatory, and pro-angiogenic properties—with biocompatible hydrogel delivery systems. We provide a comprehensive analysis of how hydrogel encapsulation addresses the critical limitations of rapid clearance and poor retention of freely administered exosomes, enabling their sustained and localized release at the wound site. Covering foundational biology, methodological strategies for exosome loading and release, troubleshooting of system optimization, and validation through preclinical and comparative studies, this review synthesizes current research to guide scientists and drug development professionals in advancing this promising cell-free therapy toward clinical application.
The Science of Healing: Unpacking MSC Exosomes and Hydrogel Matrices
The therapeutic application of Mesenchymal Stem Cell (MSC) exosomes represents a paradigm shift in regenerative medicine, offering a cell-free alternative with significant advantages for wound healing. These nano-sized extracellular vesicles (30-150 nm) function as essential mediators of the paracrine effects of MSCs, transferring bioactive molecules to recipient cells to orchestrate tissue repair [1] [2]. Exosomes derived from MSCs promote angiogenesis, modulate inflammatory responses, stimulate cell proliferation, and enhance extracellular matrix remodeling—all critical processes in wound healing [1] [3]. Compared to stem cell transplantation, exosome-based therapies demonstrate reduced risks of immune rejection and tumorigenicity while maintaining therapeutic efficacy [1]. However, a significant clinical challenge lies in their rapid clearance from wound sites, which limits their retention and sustained therapeutic action [1] [3]. Hydrogel encapsulation has emerged as a promising strategy to address this limitation, creating a protective reservoir that prolongs exosome retention and enables controlled release at the injury site [3]. To fully exploit the therapeutic potential of engineered exosome-hydrogel systems, a comprehensive understanding of exosome biogenesis and cargo sorting mechanisms is essential for developing standardized production protocols and optimizing their regenerative capabilities.
Molecular Machinery of Exosome Biogenesis
Exosome biogenesis is a sophisticated multistep process involving the formation, cargo sorting, and secretion of vesicles originating from the endosomal system. This process initiates with the inward budding of the plasma membrane to form early endosomes, which subsequently mature into late endosomes [4] [5]. During maturation, the limiting membrane of these endosomes undergoes inward invagination, generating intraluminal vesicles (ILVs) within large multivesicular bodies (MVBs) [4] [6]. The fate of these MVBs determines whether their contents are degraded or secreted; MVBs that fuse with lysosomes undergo degradation, while those that traffic to and fuse with the plasma membrane release their ILVs into the extracellular space as exosomes [4] [6]. The molecular machinery governing these processes ensures the specific packaging of cargo and directed secretion of exosomes, with particular significance for harnessing their therapeutic potential in wound healing applications.
ESCRT-Dependent and ESCRT-Independent Biogenesis Pathways
The formation of ILVs within MVBs occurs through two primary molecular mechanisms: the endosomal sorting complex required for transport (ESCRT)-dependent pathway and several ESCRT-independent pathways [6] [7]. The ESCRT machinery consists of four multi-protein complexes (ESCRT-0, -I, -II, and -III) that operate sequentially with associated ATPases and accessory proteins [6] [7].
- ESCRT-0 initiates the process by recognizing and clustering ubiquitinated cargo proteins through ubiquitin-binding domains, simultaneously binding to the lipid phosphatidylinositol 3-phosphate (PI3P) on the endosomal membrane via FYVE domains [6].
- ESCRT-I/II complexes are subsequently recruited, forming a saddle-shaped protein structure that plays a crucial role in initiating membrane deformation and facilitating the assembly of ESCRT-III [6] [7].
- ESCRT-III undergoes sequential polymerization, driving membrane constriction and fission to ultimately generate ILVs, a process completed with the assistance of the VPS4 ATPase which catalyzes ATP hydrolysis and ESCRT complex disassembly [6] [7].
Several alternative ESCRT-dependent mechanisms exist, primarily mediated by accessory proteins such as Alix and HD-PTP [6]. The Syndecan-Syntenin-Alix pathway represents a well-characterized alternative mechanism where the transmembrane proteoglycan syndecan interacts with the adaptor protein syntenin, which subsequently recruits Alix to nucleate ESCRT-III assembly independently of ubiquitination [6]. This pathway is particularly relevant for loading specific cargoes, including heparan sulfate proteoglycans and certain growth factor receptors [6].
ESCRT-independent mechanisms primarily center on lipid-driven processes, with the neutral sphingomyelinase 2 (nSMase2)-ceramide pathway being the most extensively studied [6] [7]. nSMase2 catalyzes the conversion of sphingomyelin to ceramide within the endosomal membrane, and the cone-shaped structure of ceramide molecules facilitates negative membrane curvature, promoting inward budding and ILV formation [6]. This pathway is especially important for the packaging of specific microRNAs and heat shock proteins into exosomes [6]. Additionally, tetraspanin-rich microdomains (enriched in CD63, CD9, and CD81) contribute to ESCRT-independent ILV formation by organizing membrane platforms for selective cargo clustering and vesicle budding [6] [7].
Table 1: Key Machinery in Exosome Biogenesis Pathways
| Pathway/Component | Key Elements | Primary Function | Therapeutic Significance |
|---|---|---|---|
| ESCRT-Dependent | ESCRT-0, -I, -II, -III, VPS4 ATPase | Recognizes ubiquitinated cargo; mediates membrane deformation and scission | High cargo specificity; targetable for modulating specific protein secretion |
| Syndecan-Syntenin-Alix | Syndecan, Syntenin, Alix, ESCRT-III | Ubiquitin-independent sorting of syndecans, FGFR, KRS | Important for growth factor signaling; enhanced by heparanase activity |
| nSMase2-Ceramide | Neutral sphingomyelinase 2, ceramide | Generates ceramide to induce negative membrane curvature | Crucial for RNA and lipid cargo sorting; inhibited by GW4869 |
| Tetraspanin-Dependent | CD63, CD9, CD81 | Forms microdomains for cargo clustering and membrane budding | Defines exosome subpopulations; potential for engineered targeting |
Regulation of MVB Trafficking and Secretion
Following their formation, MVBs undergo precise intracellular trafficking, primarily along microtubules, toward the plasma membrane—a process coordinated by RAB GTPases which function as molecular switches [4]. These proteins cycle between active (GTP-bound) and inactive (GDP-bound) states, regulated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs) [4]. Among the numerous RAB proteins, RAB27A and RAB27B play pivotal roles in exosome secretion, though their functions can vary by cell type [4].
- RAB27A typically localizes to MVBs and regulates their docking at the plasma membrane through interactions with effector proteins like Slp4 [4].
- RAB27B often associates with MVBs in the perinuclear region and mediates their transport along microtubules to the cell periphery via effector proteins such as Slac2b [4].
The final steps of exosome secretion involve the docking and fusion of MVBs with the plasma membrane, processes facilitated by SNARE complexes and tethering factors [4]. The cytoskeleton, particularly actin networks and microtubules, provides the structural framework and tracks for MVB transport, with molecular motors like kinesins and dyneins enabling directional movement [4].
Mechanisms of Cargo Sorting into Exosomes
The selective packaging of biomolecules into exosomes is a highly regulated process that determines the functional properties and therapeutic potential of the secreted vesicles. Exosomes carry a diverse repertoire of cargo, including proteins, lipids, nucleic acids (RNA and DNA), and metabolites [7]. The composition of exosomal cargo is dynamic and can change in response to cellular conditions and environmental stimuli, reflecting the physiological state of the parent cell [7]. This cargo-sorting mechanism is particularly relevant for MSC exosomes, as their therapeutic efficacy in wound healing depends on the specific miRNAs, growth factors, and immunomodulatory proteins they contain [1] [3].
Protein and RNA Cargo Sorting
Protein sorting into exosomes occurs through multiple mechanisms, often involving specific sorting signals or interactions with sorting machinery. Ubiquitination serves as a primary signal for ESCRT-dependent sorting of many transmembrane proteins, including growth factor receptors like EGFR [6] [7]. As described previously, the Syndecan-Syntenin-Alix pathway mediates ubiquitin-independent sorting of specific transmembrane proteins [6]. Additionally, tetraspanin networks facilitate the clustering of specific proteins (e.g., integrins, MHC molecules) into exosome-bound microdomains [6] [7]. Certain cytosolic proteins, including heat shock proteins (HSP70, HSP90) and annexins, are enriched in exosomes through interactions with lipid membranes or other sorted proteins [7].
RNA sorting into exosomes is equally selective, with specific miRNAs and other non-coding RNAs being enriched in exosomes compared to their parent cells [7]. This process involves RNA-binding proteins (RBPs) that recognize specific motifs or modifications in RNA molecules. For instance, the RBP FAN (also known as NSFL1C) binds to the 3' end of specific miRNAs and interacts with the exosomal membrane protein LC3, facilitating miRNA loading via the nSMase2-ceramide pathway [6]. Other RBPs, such as hnRNPs and MVP, have also been implicated in the selective packaging of miRNAs and other RNAs into exosomes [7]. Some RNA sequences contain EXOmotifs or zipcodes—short nucleotide sequences recognized by RBPs that direct them to exosomes [7].
Table 2: Select Cargo Molecules in MSC Exosomes and Their Roles in Wound Healing
| Cargo Type | Example Molecules | Function in Wound Healing | Sorting Mechanism |
|---|---|---|---|
| miRNA | miR-125a, miR-31, miR-192-5p | Promote angiogenesis; regulate scar formation | RBP-mediated (e.g., FAN); nSMase2-dependent |
| Growth Factors | VEGF, TGF-β, HGF | Stimulate angiogenesis; fibroblast proliferation | Syndecan-Syntenin-Alix pathway; Tetraspanin networks |
| Immunomodulatory Proteins | TSG-6, IL-10 | Polarize macrophages to M2 anti-inflammatory phenotype | ESCRT-dependent; Ubiquitin-independent mechanisms |
| Extracellular Matrix Proteins | Fibronectin, Collagen | Support cell migration; tissue structure | Lipid raft-mediated; Tetraspanin-associated |
Impact of Cellular Environment on Cargo Loading
The cellular microenvironment significantly influences exosome cargo sorting, a consideration of paramount importance for producing therapeutic MSC exosomes. Pathological conditions such as hypoxia, inflammation, and nutrient starvation can alter the expression and activity of sorting machinery components, thereby modifying the composition and function of secreted exosomes [4] [6]. For example, in cancer cells, oncogenic signaling can upregulate the Syndecan-Syntenin-Alix pathway, increasing the secretion of exosomes that promote metastasis [6]. Similarly, inflammatory cytokines can modulate the sorting of immunomodulatory miRNAs into MSC exosomes, enhancing their anti-inflammatory potential—a highly desirable characteristic for wound healing applications [1] [3]. Understanding these regulatory mechanisms provides opportunities for preconditioning MSCs during culture to tailor the therapeutic properties of their exosomes for specific wound healing indications.
Experimental Protocols for Studying Exosome Biogenesis and Cargo
Protocol: Isolation and Characterization of MSC Exosomes
This protocol describes standard methods for obtaining high-purity exosomes from MSC culture supernatants, a critical first step for both basic research and therapeutic development [5] [8].
Materials:
- Cell Culture: Mesenchymal Stem Cells (from bone marrow, adipose tissue, or umbilical cord), appropriate growth medium (e.g., DMEM/F12 with 10% FBS), exosome-depleted FBS.
- Isolation Reagents: Phosphate-buffered saline (PBS), sterile ultracentrifugation tubes.
- Characterization Reagents: Primary antibodies (anti-CD63, anti-CD81, anti-CD9, anti-TSG101, anti-Calnexin), glutaraldehyde (for TEM).
Procedure:
- Cell Culture and Conditioned Media Collection:
- Culture MSCs to 70-80% confluence in standard growth medium.
- Replace with fresh medium containing exosome-depleted FBS and culture for 24-48 hours.
- Collect conditioned media and perform sequential centrifugation: 300 × g for 10 min (remove cells), 2,000 × g for 20 min (remove dead cells), and 10,000 × g for 30 min (remove cell debris and large vesicles).
Exosome Isolation via Ultracentrifugation:
- Transfer the supernatant to ultracentrifugation tubes.
- Centrifuge at 100,000 × g for 70 minutes at 4°C.
- Carefully discard the supernatant and resuspend the exosome pellet in a suitable volume of PBS.
- Filter the suspension through a 0.22 μm filter.
- Perform a second ultracentrifugation wash under the same conditions (100,000 × g, 70 min).
- Resuspend the final purified exosome pellet in PBS and aliquot for storage at -80°C.
Exosome Characterization:
- Nanoparticle Tracking Analysis (NTA): Dilute exosomes in PBS and inject into the NTA system to determine particle size distribution and concentration.
- Transmission Electron Microscopy (TEM): Fix exosomes with glutaraldehyde, adsorb onto Formvar-carbon coated grids, negative stain with uranyl acetate, and image to confirm cup-shaped morphology.
- Western Blotting: Lyse exosomes and analyze for the presence of positive markers (CD63, CD81, TSG101) and absence of negative markers (Calnexin, GM130).
Protocol: Inhibiting Exosome Biogenesis to Confirm Functional Roles
This protocol utilizes pharmacological inhibitors to disrupt specific biogenesis pathways, allowing researchers to link exosome secretion to specific functional outcomes in wound healing assays [6].
Materials:
- Inhibitors: GW4869 (nSMase2 inhibitor), Manumycin A (RAS and exosome secretion inhibitor), DMVAA (VPS4 inhibitor).
- Cell Culture: MSC cultures, wound healing assay reagents (e.g., migration plates, angiogenesis kits).
Procedure:
- Treatment of MSCs:
- Culture MSCs to 60-70% confluence.
- Treat cells with optimized concentrations of inhibitors (e.g., 10-20 μM GW4869, 5-10 μM Manumycin A) or vehicle control (DMSO) in exosome-depleted medium for 24-48 hours.
Validation of Inhibition:
- Collect conditioned media from treated and control cells.
- Iserve exosomes using the protocol in 4.1.
- Quantify exosome yield using NTA. A significant reduction in particle count confirms effective inhibition.
Functional Wound Healing Assays:
- Collect conditioned media from inhibitor-treated and control MSCs. This media contains secreted factors but a depleted level of exosomes in the inhibitor group.
- Apply this conditioned media to in vitro wound healing models:
- Cell Migration Scratch Assay: Treat fibroblasts or keratinocytes with the conditioned media and measure the rate of gap closure in a scratch wound.
- Tube Formation Assay: Seed endothelial cells on Matrigel and treat with conditioned media. Quantify the number of tubular structures formed.
- Compare the pro-migratory and pro-angiogenic effects of media from inhibitor-treated versus control MSCs. A significant reduction in functionality with inhibitor-treated media confirms the critical role of MSC exosomes in these processes.
The Scientist's Toolkit: Key Research Reagents
Table 3: Essential Reagents for Studying Exosome Biogenesis and Function
| Reagent/Category | Specific Examples | Primary Function in Research |
|---|---|---|
| Biogenesis Inhibitors | GW4869, Manumectin A, DMVAA | Chemically disrupts specific biogenesis pathways (nSMase2, RAB27, VPS4) to study exosome function. |
| Isolation Kits | Total Exosome Isolation Kits, Size-Exclusion Chromatography Columns, PEG-based Precipitation Kits | Rapid isolation of exosomes from cell culture media or biological fluids; alternatives to UC. |
| Characterization Antibodies | Anti-CD63, Anti-CD81, Anti-CD9, Anti-TSG101, Anti-Alix, Anti-Calnexin | Confirm exosome identity via Western Blot, flow cytometry, or immuno-EM. |
| Fluorescent Tracking Dyes | PKH67, PKH26, DiI, CFSE | Label exosome membranes for uptake, tracking, and biodistribution studies in recipient cells. |
| Engineering Tools | Electroporators, Sonication Devices, Transfection Reagents (for parental cells) | Load therapeutic cargo (drugs, nucleic acids) into exosomes via exogenous or endogenous methods. |
Integration with Hydrogel Encapsulation for Wound Healing
The controlled biogenesis and cargo loading of MSC exosomes find their ultimate therapeutic application in their integration with hydrogel-based delivery systems. Hydrogels address the critical pharmacokinetic challenge of rapid exosome clearance from wound sites, providing a three-dimensional scaffold that protects exosomes and enables their sustained, localized release [9] [1] [3]. The porous structure of hydrogels can be fine-tuned to modulate diffusion rates, while their biocompatibility ensures a moist wound environment conducive to healing [3]. Integrating exosomes with hydrogels often involves simple mixing during hydrogel formation (e.g., with chitosan, hyaluronic acid) or more sophisticated methods like microfluidic encapsulation [1] [3].
The future of exosome-based wound therapies lies in engineered exosomes [10] [8]. Knowledge of biogenesis and cargo sorting enables the production of exosomes loaded with specific therapeutic molecules (e.g., growth factors, RNA interference agents) or surface-modified with targeting ligands (e.g., using peptide tags) to enhance their homing to specific wound cell types like fibroblasts or endothelial cells [10] [8]. When such precisely engineered exosomes are encapsulated within a hydrogel, the result is a sophisticated "smart" therapeutic system capable of providing localized, sustained, and targeted treatment for chronic wounds, ultimately bridging the gap between fundamental cell biology and clinical regenerative medicine.
Mesenchymal stem cell-derived exosomes (MSC-Exos) have emerged as powerful acellular nanotherapeutics in regenerative medicine, particularly in the context of wound healing and tissue repair. These nanoscale vesicles (30-150 nm in diameter) mediate the paracrine effects of their parent cells by transferring functional proteins, lipids, and nucleic acids to recipient cells [11] [12]. When encapsulated in hydrogels for sustained wound release, MSC-Exos offer significant advantages over traditional cell-based therapies, including low immunogenicity, enhanced stability, and the ability to penetrate biological barriers [11] [13]. Their therapeutic potential stems from their sophisticated mechanisms for modulating inflammation, promoting angiogenesis, and facilitating tissue remodeling—the three critical phases of wound healing.
Molecular Mechanisms of Action
Immunomodulation and Inflammation Control
MSC-Exos precisely regulate the immune response throughout the wound healing process, primarily through their microRNA cargo and surface proteins:
Macrophage Polarization: MSC-Exos modulate the balance between pro-inflammatory M1 and anti-inflammatory M2 macrophages. Depending on the wound microenvironment, they can promote polarization toward the M2 phenotype via regulation of the JAK1/STAT1/STAT6 signaling pathway and miR-146a release, reducing excessive inflammation [12]. Conversely, in fibrotic environments, they may stimulate M1 differentiation to counteract fibrosis [12].
Lymphocyte Regulation: MSC-Exos suppress aberrant adaptive immune responses by inhibiting T-cell proliferation and activity through miR-125a-3p, maintaining Th1/Th2 balance, and suppressing Th17 expansion [12]. They also inhibit B-cell proliferation and antibody production via miR-155-5p [12].
Dendritic Cell Modulation: Through release of miR-21-5p, MSC-Exos inhibit dendritic cell maturation and reduce expression of MHC-II and costimulatory molecules, thereby decreasing antigen presentation [12].
Table 1: Key Immunomodulatory Components in MSC Exosomes
| Molecular Cargo | Target Cell/Pathway | Biological Effect | Therapeutic Outcome |
|---|---|---|---|
| miR-146a | Macrophages JAK1/STAT1/STAT6 | Promotes M2 polarization | Reduces inflammation |
| miR-21-5p | Dendritic cells | Inhibits maturation | Decreases antigen presentation |
| miR-155-5p | B cells | Inhibits proliferation & antibody production | Suppresses adaptive immunity |
| miR-125a-3p | T cells | Suppresses T cell activity | Maintains Th1/Th2 balance |
| TGF-β | NK cells, SMAD2 pathway | Inhibits NK cell cytotoxicity | Reduces immune activation |
Angiogenic Programming
MSC-Exos directly address microvascular dysfunction in chronic wounds by activating multiple pro-angiogenic pathways:
Growth Factor Activation: Exosomes promote angiogenesis primarily through Vascular Endothelial Growth Factor (VEGF), FGF2, and PDGF signaling, stimulating endothelial cell proliferation, migration, and tube formation [14].
miRNA-Mediated Angiogenesis: Specific microRNAs such as miR-126 play crucial roles in enhancing angiogenic responses. MSC-Exos deliver these bioactive molecules directly to endothelial cells, activating Wnt/β-catenin, Notch, and PI3K/Akt pathways that are essential for new blood vessel formation [14].
Hypoxic Preconditioning: Under hypoxic conditions, MSC-Exos are enriched with additional angiogenic factors that prevent tissue ischemia, making them particularly effective for treating wounds with compromised blood supply [12].
Tissue Remodeling and Repair
MSC-Exos facilitate the proliferative and remodeling phases of wound healing through multiple mechanisms:
Extracellular Matrix Regulation: Exosomes modulate fibroblast activity and collagen deposition by regulating MMPs and their inhibitors, ensuring proper balance between matrix synthesis and degradation [15]. This prevents abnormal scar formation while supporting functional tissue reconstruction.
Cellular Proliferation and Migration: Through transfer of growth factors and regulatory RNAs, MSC-Exos enhance the migration and proliferation of keratinocytes, fibroblasts, and endothelial cells essential for re-epithelialization and granulation tissue formation [15].
Anti-fibrotic Effects: In conditions characterized by excessive fibrosis, MSC-Exos deliver anti-fibrotic miRNAs that suppress collagen overproduction and myofibroblast differentiation, particularly important for treating hypertrophic scars and fibrotic diseases [12].
Quantitative Analysis of MSC Exosome Effects
Table 2: Therapeutic Effects of MSC Exosomes in Wound Healing Applications
| Therapeutic Effect | Key Molecular Mediators | Experimental Evidence | Efficiency/Impact |
|---|---|---|---|
| Angiogenesis | VEGF, FGF2, miR-126, PI3K/Akt | Increased capillary density in diabetic wounds [14] | 3.7-fold reduction in amputation risk in microvascular disease [14] |
| Immunomodulation | miR-146a, miR-21-5p, TGF-β | Shift from M1 to M2 macrophages in chronic wounds [12] | Significant reduction in pro-inflammatory cytokines (TNF-α, IL-6) [12] |
| Re-epithelialization | FGFs, KGFs, IGF-1 | Accelerated keratinocyte migration and wound closure [15] | 5-fold acceleration in wound closure in preclinical models [14] |
| Anti-fibrosis | Regulatory miRNAs, TIMP1 | Reduced collagen deposition in fibrotic models [12] [15] | Improved tissue flexibility and function |
| Clinical Translation | Multiple combined mechanisms | 64 registered clinical trials for MSC-EVs [11] | Completed trials show significant wound healing progress [11] |
Experimental Protocols for MSC Exosome Research
Protocol: Isolation and Characterization of MSC Exosomes
Purpose: To isolate and characterize exosomes from mesenchymal stem cell culture supernatants for wound healing applications.
Materials:
- MSC culture (bone marrow, adipose tissue, or umbilical cord derived)
- Serum-free MSC culture medium
- Ultracentrifugation equipment
- Transmission Electron Microscope (TEM)
- Nanoparticle Tracking Analysis (NTA) system
- Western blot equipment
- Antibodies for CD63, CD81, CD9, CD73, CD90, CD105
Procedure:
- Cell Culture: Culture MSCs in serum-free medium for 48 hours to accumulate exosomes in conditioned medium.
- Differential Centrifugation:
- 300 × g for 10 min to remove cells
- 2,000 × g for 20 min to remove dead cells
- 10,000 × g for 30 min to remove cell debris
- 100,000 × g for 70 min to pellet exosomes
- Purification: Wash exosome pellet in PBS and repeat ultracentrifugation at 100,000 × g for 70 min.
- Characterization:
- Size and Concentration: Use NTA to determine particle size distribution (should be 30-150 nm) and concentration.
- Morphology: Confirm spherical morphology using TEM.
- Surface Markers: Verify presence of exosomal markers (CD63, CD81, CD9) and MSC markers (CD73, CD90, CD105) via western blot.
- Storage: Resuspend in PBS and store at -80°C until use.
Quality Control: Ensure negative staining for calnexin (non-exosomal marker) and appropriate particle-to-protein ratio.
Protocol: Hydrogel Encapsulation of MSC Exosomes
Purpose: To encapsulate MSC exosomes in hyaluronic acid hydrogel for sustained release in wound healing applications.
Materials:
- Purified MSC exosomes
- Hyaluronic acid (HA, 1-2% w/v)
- Crosslinking agent (e.g., divinyl sulfone or adipic acid dihydrazide)
- Phosphate Buffered Saline (PBS), pH 7.4
- BCA protein assay kit
- ELISA kits for exosome markers
Procedure:
- Hydrogel Preparation: Dissolve hyaluronic acid in PBS to achieve 1.5% (w/v) solution.
- Exosome Incorporation: Mix purified exosomes (50-200 μg protein equivalent) with HA solution at 4°C.
- Crosslinking: Add crosslinking agent at optimized concentration and incubate at 37°C for 2 hours to form stable hydrogel.
- Characterization:
- Rheology: Measure storage (G') and loss (G") moduli to confirm hydrogel formation.
- Release Kinetics: Immerse exosome-loaded hydrogel in PBS at 37°C with gentle shaking. Collect supernatant at predetermined time points and quantify exosome release using BCA protein assay and CD63 ELISA.
- Bioactivity Assessment: Test released exosomes in endothelial tube formation assay to confirm retained angiogenic activity.
Optimization Notes: Adjust crosslinking density to achieve desired release profile (typically sustained over 7-14 days). Sterilize final product via gamma irradiation for in vivo applications.
Protocol: Functional Validation of MSC Exosome Bioactivity
Purpose: To validate the functional capabilities of MSC exosomes in modulating inflammation, angiogenesis, and tissue remodeling.
Materials:
- Isolated MSC exosomes
- Human umbilical vein endothelial cells (HUVECs)
- Macrophage cell line (e.g., THP-1)
- Fibroblast cell line (e.g., HDF)
- Matrigel for tube formation assay
- Transwell migration chambers
- ELISA kits for TNF-α, IL-10, VEGF
- RNA extraction kit and qPCR equipment
Angiogenesis Assay:
- Endothelial Tube Formation: Seed HUVECs on Matrigel-coated plates. Treat with MSC exosomes (10-50 μg/mL). After 4-8 hours, quantify tube formation by measuring total tube length and branch points.
- Endothelial Migration: Use scratch wound assay or Transwell chambers to assess HUVEC migration toward exosome-treated conditioned medium.
Immunomodulation Assay:
- Macrophage Polarization: Differentiate THP-1 cells into M0 macrophages, then treat with LPS/IFN-γ for M1 or IL-4/IL-13 for M2 polarization in presence of MSC exosomes.
- Cytokine Profiling: After 24-48 hours, measure TNF-α, IL-6 (M1 markers) and IL-10, TGF-β (M2 markers) using ELISA.
- Surface Marker Analysis: Assess CD86 (M1) and CD206 (M2) expression by flow cytometry.
Tissue Remodeling Assay:
- Fibroblast Function: Treat fibroblasts with exosomes and assess:
- Collagen production (Sirius Red staining)
- MMP expression (zymography)
- Migration capacity (scratch assay)
- Gene Expression: Analyze fibrotic markers (α-SMA, collagen I, collagen III) via qPCR.
Data Interpretation: Compare results with appropriate controls (PBS-treated) and calculate statistical significance. Effective MSC exosomes should enhance tube formation, promote M2 macrophage polarization, and modulate fibroblast collagen production.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for MSC Exosome Studies in Wound Healing
| Reagent Category | Specific Examples | Function/Application | Considerations |
|---|---|---|---|
| Isolation Kits | Total Exosome Isolation Kit, miRCURY Exosome Kit | Rapid exosome isolation from conditioned medium | Compare yield/purity with ultracentrifugation; may vary by MSC source |
| Characterization Antibodies | Anti-CD63, CD81, CD9, CD73, CD90, CD105 | Confirm exosome identity and MSC origin | Include negative markers (calnexin) for purity assessment |
| Hydrogel Components | Hyaluronic acid, chitosan, PEG-based polymers | Create sustained release delivery systems | Adjust crosslinking density to control release kinetics |
| Angiogenesis Assays | Matrigel, HUVECs, VEGF ELISA | Quantify pro-angiogenic potential | Include positive (VEGF) and negative controls |
| Immunomodulation Assays | THP-1 cells, LPS/IFN-γ, IL-4/IL-13, cytokine ELISA | Assess macrophage polarization | Characterize both M1 and M2 markers for balanced assessment |
| Cell Migration Assays | Transwell chambers, scratch assay reagents | Evaluate cellular migration enhancement | Standardize initial wound size and serum conditions |
| qPCR Components | Primers for miR-126, miR-146a, inflammatory genes | Analyze miRNA delivery and gene expression changes | Normalize using appropriate housekeeping genes |
MSC exosomes represent a sophisticated acellular nanotherapeutic platform that coordinately modulates inflammation, angiogenesis, and tissue remodeling through delivery of complex molecular cargo. Their encapsulation in hydrogels for sustained wound release addresses critical challenges in regenerative medicine, particularly for chronic wounds characterized by microvascular dysfunction and persistent inflammation [14] [11]. The mechanistic insights and standardized protocols provided in this application note establish a foundation for reproducible research and development in this rapidly advancing field.
Future directions include engineering exosomes for enhanced target specificity, developing combination therapies with growth factors or pharmaceuticals, and establishing scalable production methods for clinical translation [11] [13]. As research progresses, MSC exosome-hydrogel composites hold significant promise for revolutionizing the treatment of complex wounds and other ischemic conditions.
Hydrogels are highly hydrophilic, three-dimensional network structures composed of cross-linked polymers that can swell in water while retaining a large volume of water without dissolving [16]. These biopolymer networks serve as foundational scaffolds in tissue engineering and regenerative medicine, providing a protective microenvironment that mimics the natural extracellular matrix (ECM). Their unique physical and chemical properties make them particularly valuable for the controlled delivery of therapeutic agents, including mesenchymal stem cell-derived exosomes (MSC-Exos), which require sustained release and protection from rapid clearance to effectively promote tissue repair [17] [16]. The biocompatibility, tunable mechanical properties, and capacity for controlled bioactive molecule release position hydrogels as essential components in advanced wound healing strategies, particularly for complex diabetic wounds characterized by prolonged inflammation, oxidative stress, and impaired angiogenesis [18] [17].
The structural integrity of hydrogels arises from physical or chemical cross-linking of polymer chains, creating a mesh-like architecture with defined porosity that can be engineered to control the diffusion and release kinetics of encapsulated therapeutics [19]. Natural polymer-based hydrogels, such as those derived from recombinant human collagen (RHC) or hyaluronic acid, offer enhanced biocompatibility and biological recognition sites that support cellular activities and tissue integration [9] [19]. When integrated with MSC-Exos, hydrogels form a composite therapeutic system that synergistically combines the structural support and controlled release capabilities of the hydrogel with the multifaceted regenerative signals of the exosomes, establishing a paradigm-shifting approach for managing diabetic complications and other chronic wounds [17].
Key Characteristics of Hydrogel Networks
Table 1: Fundamental Properties of Hydrogel Networks for Therapeutic Applications
| Property | Structural Basis | Functional Significance | Influence on Exosome Delivery |
|---|---|---|---|
| Porous Structure | Interconnected 3D network of polymer chains with tunable pore size (micro to nano scale) [16] [19] | Enables nutrient/waste diffusion and cell infiltration while controlling therapeutic agent release [16] | Determines exosome encapsulation efficiency and release kinetics; smaller pores prolong release [19] |
| Hydration Capacity | Highly hydrophilic polymer chains with water absorption capacity up to 90% of total mass [16] | Maintains moist wound environment; facilitates metabolite transport; mimics native tissue conditions [17] | Preserves exosome bioactivity in aqueous environment; prevents premature degradation [17] |
| Mechanical Properties | Cross-linking density and polymer composition (compressive stress: 47.9-136.8 kPa adjustable) [19] | Provides structural support to wound bed; mechanical cues influence cell behavior and tissue regeneration [19] | Higher cross-linking density creates more stable exosome reservoir with slower release profile [18] [19] |
| Biocompatibility | Natural polymer composition (e.g., recombinant human collagen) with minimal immune reaction [19] | Enables safe clinical application; supports cell adhesion and proliferation without cytotoxicity [19] | Maintains exosome membrane integrity and biological function; ensures therapeutic efficacy [17] [19] |
| Tunable Degradation | Engineered cross-linkers (enzymatically cleavable, photodegradable) with controllable kinetics [17] | Matches degradation rate to tissue regeneration timeline; prevents foreign-body reactions [17] | Synchronizes exosome release with healing phases; provides sustained bioactive cargo delivery [18] [17] |
Quantitative Performance Data of Hydrogel-Exosome Systems
Table 2: Experimental Performance Metrics of Advanced Hydrogel-Exosome Formulations
| Hydrogel System | Exosome Source | Release Kinetics | Therapeutic Outcomes | Key Mechanisms |
|---|---|---|---|---|
| Methacrylated ADM (Exo@AMCN) [18] [20] | Human umbilical cord MSC (hUCMSC-Exo) | Controlled release over 2-7 days via adjustable cross-linking density [18] | Residual wound area reduced to 1.07 ± 1.27% in 14 days; >85% antibacterial efficacy [18] [20] | Macrophage polarization to anti-inflammatory M2 phenotype; enhanced angiogenesis and collagen deposition [18] |
| Recombinant Human Collagen (RHCMA) [19] | ucMSC-exos, BMSC-exos, ADSC-exos | 56.27 ± 4.48% release in first 12 h; 92.27 ± 3.19% after 48 h (10% RHCMA) [19] | ucMSC-exos@RHCMA showed best healing with accelerated inflammatory resolution and angiogenesis [19] | ucMSC-exos enhanced macrophage regulation, oxidative stress reduction, and collagen formation [19] |
| Protein-based Q5 Hydrogel [21] | Adipose-derived MSC exosomes | Sustained release via topical application without injections [21] | Significant reduction in healing time vs. exosome injection in diabetic mouse models [21] | Upper critical solution temperature (UCST) gelation provides mechanical strength for localized exosome delivery [21] |
| GelMA-ZIF-8 Composite [22] | MSC-Exos (miR-23a-3p enriched) | Sustained release of exosomes and zinc ions [22] | Enhanced new bone formation and angiogenesis with maintained low inflammation [22] | miR-23a-3p targets PTEN to activate AKT pathway for osteogenesis; ZIF-8 induces M2 polarization [22] |
Experimental Protocols
Protocol: Fabrication of Sprayable Photocrosslinkable Hydrogel for Exosome Delivery
This protocol describes the synthesis of a methacrylated acellular dermal matrix (ADM) hydrogel for controlled co-delivery of hUCMSC-derived exosomes and therapeutic compounds, adapted from studies demonstrating significant wound healing efficacy in diabetic models [18] [20].
Materials:
- Methacrylation-modified acellular dermal matrix (ADM)
- Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) photoinitiator
- hUCMSC-derived exosomes (100-150 nm diameter, characterized by TEM and DLS)
- β-cyclodextrin-borneol inclusion complexes (CN)
- 405 nm blue light source
- Phosphate buffered saline (PBS, pH 7.4)
- Sterile spray applicator
Procedure:
- Hydrogel Precursor Preparation:
- Dissolve methacrylated ADM in PBS at 10-15% (w/v) concentration
- Add LAP photoinitiator at 0.05-0.1% (w/v) and mix until fully dissolved
- Incorporate hUCMSC-Exos (50-100 μg/mL) and β-cyclodextrin-borneol complexes (1-2% w/v) into the precursor solution under gentle agitation
Application and Cross-linking:
- Transfer the precursor solution to a sterile spray applicator
- Apply evenly to the wound surface using a sweeping motion to cover the entire area
- Immediately expose to 405 nm blue light at 5-15 mW/cm² intensity for 10-300 seconds
- Adjust exposure duration based on desired cross-linking density: shorter times (10-30 s) for softer gels with faster release; longer times (120-300 s) for denser gels with prolonged release
Characterization and Validation:
- Verify gel formation through visual inspection and mechanical testing
- Assess exosome release kinetics by measuring fluorescently labeled exosomes in PBS supernatant over 7 days
- Confirm antibacterial activity against common pathogens (S. aureus, P. aeruginosa) using zone of inhibition assays
Protocol: Recombinant Human Collagen Hydrogel for Comparative Exosome Studies
This methodology enables direct comparison of therapeutic efficacy between different MSC-derived exosomes encapsulated within a tunable recombinant human collagen hydrogel platform, supporting identification of optimal exosome sources for specific wound healing applications [19].
Materials:
- Recombinant human collagen (RHC)
- Methacrylate anhydride (MA)
- Photoinitiator (Irgacure 2959 or similar)
- MSC-derived exosomes (ucMSC-exos, BMSC-exos, ADSC-exos)
- NMR spectrometer for chemical validation
- Scanning electron microscope
- UV light source (365 nm, 5-10 mW/cm²)
- CCK-8 assay kit for cytotoxicity testing
Procedure:
- RHCMA Synthesis:
- Modify RHC macromolecular chains with methacrylate anhydride via condensation reaction
- Confirm successful modification using ¹H NMR spectroscopy: verify signals at 5.4 and 5.6 ppm (acrylic protons) and 1.8 ppm (methyl groups in methacrylate)
- Prepare pre-gel solutions at varying concentrations (10%, 12.5%, 15%, 17.5% w/v) in PBS
Hydrogel Characterization:
- Expose pre-gel solutions to UV light (365 nm) for 5-10 minutes to initiate cross-linking
- Analyze microstructure using SEM: confirm decreasing pore size with increasing RHCMA concentration
- Perform compression testing: expected maximum compressive stress of 47.9 ± 6.6 kPa for 10% concentration to 136.8 ± 9.6 kPa for 17.5% concentration
- Determine swelling ratio: 14.5 ± 0.6 for 10% concentration to 7.3 ± 1.0 for 17.5% concentration after 60 minutes immersion
Exosome Encapsulation and Release Profiling:
- Isolate exosomes from ucMSCs, BMSCs, and ADSCs using ultracentrifugation method
- Characterize exosomes by TEM (bilayer vesicle structure, 100-110 nm diameter) and dynamic light scattering
- Incorporate exosomes into 10% RHCMA pre-gel solution at 50-100 μg/mL concentration
- Conduct in vitro release studies using DID-fluorescently labeled exosomes in PBS at 37°C over 72 hours
- Sample release medium at predetermined intervals (1, 3, 6, 12, 24, 48, 72 h) and measure fluorescence intensity
Signaling Pathways in Hydrogel-Exosome Mediated Healing
The diagram above illustrates the coordinated molecular and cellular pathways through which hydrogel-loaded MSC exosomes promote tissue regeneration. The sustained release of exosomes from the hydrogel matrix enables multi-phase regulation of the healing process, particularly critical in diabetic wounds characterized by chronic inflammation [17]. Key exosomal miRNAs including miR-23a-3p and miR-219-5p drive the polarization of macrophages from pro-inflammatory M1 to anti-inflammatory M2 phenotypes, creating a conducive microenvironment for regeneration [16] [22]. Simultaneously, exosome-mediated activation of VEGF/VEGFR signaling pathways stimulates angiogenesis, while PTEN targeting and AKT pathway activation promotes osteogenic differentiation for bone repair [17] [22]. These parallel processes collectively enhance extracellular matrix remodeling and collagen deposition, addressing the fundamental pathophysiological barriers to healing in chronic wounds.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Essential Research Reagents for Hydrogel-Exosome Formulation and Analysis
| Reagent/Category | Specific Examples | Function & Application | Experimental Notes |
|---|---|---|---|
| Hydrogel Polymers | Recombinant Human Collagen (RHC), Methacrylated ADM, GelMA, Hyaluronic Acid [9] [18] [19] | Provides 3D scaffold structure; enables tunable mechanical properties and degradation kinetics | Recombinant human collagen offers superior biocompatibility; methacrylation allows photopolymerization [19] |
| Cross-linking Agents | Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP), Irgacure 2959, Methacrylate Anhydride [18] [20] [19] | Initiates photopolymerization; controls cross-linking density and subsequent release kinetics | LAP enables visible light cross-linking (405 nm); cross-linking time (10-300 s) controls degradation [18] |
| Exosome Sources | ucMSC-Exos, BMSC-Exos, ADSC-Exos [16] [19] | Provides therapeutic cargo (miRNAs, proteins, lipids) for immunomodulation, angiogenesis, and tissue repair | ucMSC-Exos show superior anti-inflammatory effects; ADSC-Exos enhance angiogenesis [19] |
| Characterization Tools | Transmission Electron Microscopy, Dynamic Light Scattering, NMR Spectroscopy [16] [19] | Validates exosome morphology/size and hydrogel chemical modification | Exosomes should show bilayer structure, 100-110 nm size; NMR confirms methacrylation [19] |
| Therapeutic Adjuvants | β-cyclodextrin-borneol complexes, ZIF-8 [18] [22] | Enhances antibacterial activity, ROS scavenging, and immunomodulation in composite formulations | Borneol complexes provide >85% antibacterial efficacy; ZIF-8 induces M2 macrophage polarization [18] [22] |
| Release Tracking | DID fluorescent dye, Fluorescence spectroscopy [19] | Quantifies exosome release kinetics from hydrogel systems | 10% RHCMA releases 56.27±4.48% exosomes in first 12h; reaches 92.27±3.19% by 48h [19] |
Advanced Formulation Design Workflow
The workflow illustrates the systematic approach to developing advanced hydrogel-exosome formulations, beginning with careful selection of both polymer base and exosome source based on target application [19]. Chemical modification through methacrylation introduces photopolymerizable groups that enable subsequent light-controlled cross-linking, while parallel exosome isolation and characterization ensures therapeutic cargo quality and consistency [18] [19]. The encapsulation process employs physical mixing to maintain exosome integrity, followed by precision cross-linking that determines subsequent release kinetics and mechanical properties [19]. Comprehensive release profiling validates the sustained delivery capabilities of the system, while in vitro and in vivo efficacy testing confirms therapeutic performance through assessment of macrophage polarization, angiogenesis, collagen deposition, and wound closure metrics [18] [19]. This integrated approach enables researchers to tailor hydrogel-exosome systems for specific clinical requirements, particularly for complex wound healing scenarios where controlled spatiotemporal delivery of regenerative signals is essential for optimal outcomes.
Chronic wounds, characterized by a failure to proceed through the normal, orderly, and timely healing process within three months, represent a severe and growing global health challenge [23]. These wounds, including diabetic foot ulcers, venous leg ulcers, and pressure injuries, are defined by a pathological microenvironment featuring prolonged inflammation, excessive reactive oxygen species (ROS), impaired angiogenesis, slowed cell proliferation, and delayed extracellular matrix (ECM) remodeling [23]. Current clinical strategies, such as negative pressure wound therapy, antibiotic-based infection control, and wound debridement, primarily target local wound conditions and offer only short-term relief, failing to achieve sustained functional regeneration [24].
Cell-based therapies, particularly those utilizing mesenchymal stem cells (MSCs), have emerged as promising alternatives due to their ability to suppress inflammation, stimulate angiogenesis, and promote cellular proliferation [24]. However, the therapeutic potential of MSCs is significantly limited by low post-transplantation survival rates, risks of immune rejection, and potential tumorigenicity [16] [24]. Consequently, research attention has shifted toward the paracrine mechanisms of MSCs, particularly their secreted exosomes, as a cell-free therapeutic alternative [24]. Exosomes are nano-sized extracellular vesicles (30-150 nm in diameter) secreted by all cell types, consisting of a phospholipid bilayer that carries bioactive molecules including proteins, lipids, mRNAs, and miRNAs [24]. These vesicles facilitate intercellular communication and contribute to tissue regeneration by exerting anti-inflammatory effects, promoting angiogenesis, and supporting extracellular matrix remodeling [24].
Despite their therapeutic potential, standalone exosome therapies face significant delivery challenges, including rapid clearance from the target site, enzymatic degradation, and poor retention in irregular wound geometries [16] [17]. This application note examines the synergistic rationale for combining exosomes with hydrogel-based delivery systems to overcome these limitations and enhance therapeutic outcomes in wound care.
Hydrogel-Exosome Synergy: Comparative Advantages and Mechanisms
The integration of exosomes within hydrogel systems creates a synergistic therapeutic platform that addresses the critical limitations of standalone exosome therapies while amplifying their regenerative potential. The comparative advantages of this combination are substantial and multifaceted.
Table 1: Comparative Analysis of Standalone Exosomes versus Hydrogel-Encapsulated Exosomes for Wound Therapy
| Characteristic | Standalone Exosomes | Hydrogel-Exosome Composite |
|---|---|---|
| Retention Time | Rapid clearance from administration site [16] | Sustained release over extended periods (e.g., 72-hour VEGF delivery) [17] |
| Bioactivity Protection | Susceptible to enzymatic degradation [17] | Enhanced stability and maintained biological activity [25] |
| Spatial Control | Limited localization at wound site | Prolonged retention at targeted tissues [16] |
| Therapeutic Efficacy | Suboptimal due to rapid clearance | Enhanced therapeutic efficacy; ~30% increased wound healing rate in rodent models [17] |
| Mechanical Properties | No structural support | Provides conducive 3D environment for cell regeneration [16] |
| Adaptation to Wound Bed | Limited | Conformal encapsulation adapting to wound cavity geometry [17] |
The fundamental mechanisms underlying the hydrogel-exosome synergy operate through three primary delivery strategies:
- In situ hybrid cross-linking for stimuli-triggered gelation and cavity-conformal encapsulation [17]
- Post-preloading cross-linking for covalent exosome-polymer integration [17]
- Physical adsorption exploiting hydrogel swelling dynamics to control exosome release [17]
These mechanisms collectively orchestrate spatiotemporal exosome release, bioactive cargo protection, and bidirectional molecular crosstalk, thereby establishing a paradigm-shifting approach to overcoming the enzymatic degradation, rapid clearance, and irregular tissue geometries inherent to diabetic complications and other chronic wounds [17].
Experimental Protocols: Hydrogel-Exosome Formulation and Evaluation
Protocol: Development of Injectable Hyaluronic Acid Hydrogel Loaded with MSC-Derived Exosomes
This protocol outlines the methodology for creating an injectable hyaluronic acid hydrogel incorporating mesenchymal stem cell (MSC)-derived exosomes, adapted from recent research on enhanced chronic wound healing [9].
Materials:
- Hyaluronic acid (HA, molecular weight: 100-500 kDa)
- MSC-derived exosomes (concentration: 1-5 mg/mL in PBS)
- Crosslinking agent (e.g., divinyl sulfone or adipic acid dihydrazide)
- Phosphate buffered saline (PBS, pH 7.4)
- Sterile filtration units (0.22 μm)
Procedure:
- Hyaluronic Acid Modification:
- Dissolve hyaluronic acid in PBS to achieve a 2% (w/v) solution.
- Modify HA with crosslinkable functional groups (e.g., methacrylate groups for photopolymerization) by reacting with glycidyl methacrylate (0.1:1 molar ratio) under gentle stirring for 12 hours at 4°C.
- Purify via dialysis against distilled water for 48 hours and lyophilize.
Exosome Isolation and Characterization:
- Culture MSCs in serum-free media for 48 hours.
- Collect conditioned media and isolate exosomes using differential ultracentrifugation: 300 × g for 10 min, 2,000 × g for 20 min, 10,000 × g for 30 min, followed by 100,000 × g for 70 min.
- Characterize exosomes using nanoparticle tracking analysis, transmission electron microscopy, and Western blotting for CD63, CD81, and TSG101 markers [16].
Hydrogel-Exosome Composite Formation:
- Reconstitute modified HA in PBS to form a 3% (w/v) solution.
- Mix MSC-derived exosomes (final concentration: 100-500 μg/mL) with the HA solution.
- Initiate crosslinking by adding the crosslinking agent (0.05:1 molar ratio to HA repeating units) and maintain at 37°C for 60 minutes.
- The resulting hydrogel should exhibit injectability through an 18-22 gauge needle.
Quality Control:
- Assess gelation time via vial tilting method.
- Evaluate rheological properties using oscillatory shear rheometry.
- Determine exosome encapsulation efficiency via BCA protein assay on supernatant.
Protocol: In Vitro Assessment of Bioactivity and Release Kinetics
This protocol details methods for evaluating the therapeutic functionality of hydrogel-released exosomes, critical for establishing dosage and release parameters.
Materials:
- Human dermal fibroblasts (HDFs)
- Human umbilical vein endothelial cells (HUVECs)
- Macrophage cell line (RAW 264.7)
- Cell culture media (DMEM, EGM-2)
- Transwell migration chambers
- Tube formation assay kit (e.g., Cultrex Basement Membrane Extract)
- ELISA kits for VEGF, IL-10, TNF-α
Procedure:
- Release Kinetics Study:
- Place 1 mL of exosome-loaded hydrogel in 5 mL of PBS at 37°C with gentle shaking.
- Collect release medium (200 μL) at predetermined time points (1, 3, 6, 12, 24, 48, 72 hours) and replace with fresh PBS.
- Quantify exosome release using micro-BCA protein assay or exosome-specific ELISA.
Fibroblast Migration Assay:
- Culture HDFs to 90% confluence and create a scratch wound using a 200 μL pipette tip.
- Treat with: (1) hydrogel-released exosomes, (2) fresh exosomes, (3) control media.
- Image at 0, 12, and 24 hours and calculate migration area using ImageJ software.
Angiogenic Potential Assessment:
- Seed HUVECs (1 × 10^4 cells/well) on Basement Membrane Extract.
- Treat with conditioned media from release study.
- After 6 hours, quantify tube formation by measuring total tube length and branch points.
Macrophage Polarization Study:
- Differentiate RAW 264.7 cells to M1 phenotype using LPS (100 ng/mL) and IFN-γ (20 ng/mL).
- Treat with hydrogel-released exosomes for 48 hours.
- Analyze M2 markers (CD206, Arg-1) via flow cytometry and ELISA for IL-10.
Therapeutic Mechanisms and Signaling Pathways
The therapeutic efficacy of exosome-loaded hydrogels in wound healing is mediated through multiple interconnected mechanisms that target key pathological processes in chronic wounds. The following diagram illustrates the primary signaling pathways through which MSC-derived exosomes encapsulated in hydrogels promote wound healing:
The molecular mechanisms illustrated above translate to measurable improvements in wound healing parameters. Preclinical studies have demonstrated that hypoxia-pretreated adipose-derived stem cell (ADSC)-derived exosome-embedded hydrogels increased the wound healing rate by approximately 30% and enhanced angiogenesis in rodent models [17]. The hydrogel platform provides programmable release kinetics, enabling 72-hour sustained VEGF delivery in vitro, and facilitates multifunctional regulation of the inflammatory microenvironment through coordinated antioxidant, immunomodulatory, and pro-angiogenic activities [17].
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful implementation of hydrogel-exosome wound therapy research requires specific materials and characterization tools. The following table details essential research reagent solutions for developing and evaluating these therapeutic systems.
Table 2: Essential Research Reagents for Hydrogel-Exosome Wound Therapy Development
| Category | Specific Examples | Research Function | Key Characteristics |
|---|---|---|---|
| Hydrogel Polymers | Hyaluronic acid [9], Gelatin methacryloyl (GelMA) [26], Chitosan [16], Dopamine-modified polymers [26] | 3D scaffold providing structural support and controlled release | Biocompatibility, tunable mechanical properties, biodegradability |
| Exosome Sources | MSC-derived exosomes [9], ADSC-derived exosomes [16], Plant exosomes (e.g., Momordica charantia) [26] | Primary therapeutic cargo delivering bioactive molecules | Cell-specific miRNA profiles, regenerative capacity, anti-inflammatory activity |
| Characterization Tools | Nanoparticle tracking analysis [16], Transmission electron microscopy [16], Western blot (CD63, CD81, TSG101) [16] | Verification of exosome identity, size, and concentration | Accurate quantification, morphological assessment, marker confirmation |
| Functional Assays | Tube formation assay (HUVECs) [24], Scratch wound assay [24], Macrophage polarization flow cytometry (CD206, Arg-1) [27] | Assessment of angiogenic, migratory, and immunomodulatory potential | Quantification of biological activity, mechanism validation |
| Animal Models | Diabetic mouse models (db/db or STZ-induced) [17], Full-thickness excisional wounds [26] | Preclinical evaluation of therapeutic efficacy | Pathologically relevant microenvironment, translational predictive value |
The selection of appropriate materials should be guided by the specific research objectives. For instance, GelMA and dopamine-based hydrogels offer superior antioxidant properties beneficial for diabetic wounds [26], while hyaluronic acid systems provide excellent biocompatibility and tunable physical characteristics [9]. Similarly, exosomes from different cellular sources exhibit distinct miRNA profiles and functional properties, enabling researchers to select exosomes with specific therapeutic activities aligned with their targeted wound healing applications.
The integration of exosomes within hydrogel delivery systems represents a paradigm shift in wound care therapeutics, effectively addressing the critical limitations of standalone exosome therapies while amplifying their regenerative potential through sustained, localized delivery. The synergistic combination leverages the high bioactivity and biocompatibility of exosomes with the protective and retention capabilities of hydrogels, creating a therapeutic platform capable of modulating the complex wound microenvironment through multiple coordinated mechanisms.
Future research directions should focus on personalizing exosome-hydrogel formulations using disease-stage-adjusted protocols that integrate diabetes subtypes, complication severity, and immune-genetic profiles for precision medicine [17]. Additional promising avenues include diversifying exosome sources using tissue-specific progenitors to enhance angiogenic and anti-inflammatory bioactivity [17], and engineering 3D-printed patient-specific hydrogels with lesion-matching porosity via digital light processing for anatomical precision [17]. The development of biodegradable hydrogels with tunable degradation kinetics via enzymatically cleavable crosslinkers will help prevent foreign-body reactions while ensuring complete exosome release [17].
As research in this field advances, hydrogel-exosome therapies hold tremendous promise for transforming the clinical management of chronic wounds, potentially offering solutions for millions of patients worldwide who currently lack effective treatment options. The continued refinement of these systems through rigorous preclinical evaluation and innovative bioengineering approaches will be essential for translating this promising technology into clinical practice.
Building the Delivery System: Methods for Loading and Characterizing Exosome-Laden Hydrogels
The isolation and characterization of exosomes, small extracellular vesicles (sEVs) with a size range of 30 to 150 nm, is a critical foundation for research in therapeutic delivery systems [28] [16]. Within the context of hydrogel encapsulation of mesenchymal stem cell (MSC) exosomes for sustained wound release, obtaining a pure and functionally intact exosome population is paramount [16]. These vesicles mirror the molecular composition of their parent cells, making them invaluable couriers of bioactive molecules that can promote cell proliferation, differentiation, and tissue regeneration [28] [16]. This application note provides detailed protocols for major exosome isolation techniques, with a focus on ultracentrifugation, and outlines the principal characterization methods—Transmission Electron Microscopy (TEM), Nanoparticle Tracking Analysis (NTA), and Western Blot—to ensure researchers can reliably isolate and validate exosomes for downstream applications in regenerative medicine.
Major Exosome Isolation Protocols
Selecting an appropriate isolation method is crucial and depends on experimental goals, sample type, and the required balance between yield, purity, and scalability [28]. The following section details the most common techniques.
Differential Ultracentrifugation
Differential ultracentrifugation (UC) is the most established exosome isolation protocol [28]. It involves sequential centrifugation steps to remove cells, apoptotic debris, and larger vesicles, ultimately pelleting exosomes at high forces (typically greater than 100,000 × g) [28] [29]. While considered a gold standard for its high purity, the protocol is time-consuming and requires specialized equipment [29]. Recent comparative studies show that UC yields a medium particle count but achieves high purity, as indicated by a high particle-to-protein ratio [29].
Size-Exclusion Chromatography (SEC)
SEC separates exosomes based on their size and hydrodynamic properties using a porous column [28] [29]. Larger particles, such as exosomes, are eluted first, while smaller proteins and contaminants are retained in the pores. This method is highly reproducible, maintains exosome structural integrity, and is suitable for sensitive downstream analyses [28]. However, it may be less effective for complex biological fluids and can exhibit variability in fraction-wise concentration distribution across different sample types [29].
Precipitation-Based Methods
Precipitation protocols use reagents, such as polyethylene glycol (PEG), to force exosomes out of solution [28]. These methods are fast, require only a standard centrifuge, and yield high particle counts [28] [29]. The main drawback is lower purity due to co-precipitation of non-vesicular contaminants like lipoproteins and protein aggregates [28] [29]. A novel, efficient cocktail strategy integrating chemical precipitation with a two-step ultrafiltration process (CPF) has been developed to enhance purity while maintaining ease of use [29].
Immunoaffinity Capture
This technique employs antibodies targeting specific exosomal surface markers (e.g., CD9, CD63, CD81) for subtype-specific isolation [28] [30]. It provides very high selectivity and is ideal for studying exosome subpopulations. Its limitations include limited throughput, high cost, and the requirement for specific antibodies [28]. Advanced applications of this principle use genetic engineering to label exosomes from specific cell types in vivo for precise isolation from complex tissues [30].
Comparative Performance of Isolation Methods
The table below summarizes the key performance metrics of the primary isolation techniques, aiding in method selection.
Table 1: Comparative performance metrics for exosome isolation protocols
| Method | Purity | Yield | Scalability | Instrumentation | Time Requirement |
|---|---|---|---|---|---|
| Ultracentrifugation | High | Medium | Medium | Ultracentrifuge | High (Time-consuming) |
| SEC | Medium-High | Medium | High | Chromatography system | Medium |
| Precipitation | Low | High | High | Centrifuge | Low (Rapid) |
| Immunoaffinity Capture | Very High | Low | Low | Antibody-conjugated surfaces | Medium |
Table 2: Quantitative comparison of sEVs isolated from cell culture media using different methods (adapted from [29])
| Method | Particle Concentration (Particles/mL) | Mean Particle Size (nm) | Particle-to-Protein Ratio |
|---|---|---|---|
| CP (Precipitation) | 1.46E+10 | 76.13 ± 4.4 | Low |
| CPF (Precipitation + Filtration) | Successively higher than UC | 84.97 ± 8.2 (SEC example) | Higher than CP |
| UC | 1.3E+09 | 88.13 ± 5.1 | High |
| SEC | Successively lower than CP | 95.5 ± 8.9 | High |
Essential Characterization Techniques
Validating isolated exosomes is critical to confirm their identity, purity, and integrity. According to MISEV guidelines, characterization should combine complementary techniques [30] [31].
Nanoparticle Tracking Analysis (NTA)
NTA determines particle size distribution and concentration by tracking the Brownian motion of individual particles in a suspension [28] [29]. It is a vital tool for quantifying exosome yield and confirming the isolated population falls within the expected 30-200 nm size range [29]. Studies consistently show differences in particle concentration and size distribution based on the isolation method used [29].
Transmission Electron Microscopy (TEM)
TEM provides high-resolution imaging of exosome morphology and ultrastructure [29] [31]. Properly isolated exosomes appear as round, cup-shaped vesicular structures with a visible lipid bilayer when visualized via negative stain TEM [29]. It is particularly useful for confirming the presence of a lipid bilayer and the absence of significant non-vesicular contaminants [29]. Recent studies have introduced (semi-)automated ImageJ-based algorithms to streamline the quantification of EV diameter from TEM images, enhancing objectivity and efficiency [31].
Western Blot
Western Blot analysis is used to detect the presence of specific exosomal protein markers, confirming the vesicular origin of the preparation [29]. Key positive markers include tetraspanins (CD9, CD63, CD81) and endosomal-related proteins (TSG101, Alix) [30] [29]. The absence of negative markers, such as Grp94 (endoplasmic reticulum protein) or calnexin, should also be confirmed to rule out contamination from other cellular compartments [29]. Robust bands for CD63 (between 30-60 kDa), CD9 (around 50 kDa), and TSG101 (around 44 kDa) are indicative of successful isolation [29].
Experimental Workflow and Signaling Pathways
The following diagrams, generated with Graphviz, illustrate the core experimental workflow for exosome isolation and application, as well as the functional role of MSC exosomes in wound healing.
Figure 1: Integrated workflow for MSC exosome isolation and hydrogel application for wound healing.
Figure 2: MSC exosome-mediated activation of key signaling pathways in recipient cells at the wound site.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential materials and reagents for exosome isolation and characterization
| Item | Function/Application | Key Considerations |
|---|---|---|
| Polyethylene Glycol (PEG) | Precipitates exosomes from solution in precipitation-based kits [29]. | Enables high yield but may co-precipitate contaminants; requires purity validation [29]. |
| Anti-Tetraspanin Antibodies (CD63, CD81, CD9) | Immunoaffinity capture and Western Blot validation of exosomes [30] [29]. | Critical for subtype-specific isolation and confirming exosomal identity per MISEV guidelines [30]. |
| Protein A/G Agarose Beads | Used in conjunction with antibodies for immunoprecipitation of specific EV subpopulations [31]. | Essential for pulldown assays; requires blocking with BSA to reduce non-specific binding [31]. |
| Total Exosome Isolation Reagent | Commercial precipitation-based solution for isolating exosomes from serum or plasma [31]. | Streamlined, rapid alternative to UC; suitable for clinical samples [31]. |
| Size-Exclusion Chromatography (SEC) Columns | Isolation of exosomes based on size and hydrodynamic radius [28] [29]. | Maintains vesicle integrity and biological activity; effective for removing soluble proteins [29]. |
Chronic wounds, characterized by a failure to proceed through an orderly and timely healing process, represent a significant and growing clinical burden globally. It is predicted that 20–60 million people worldwide will be affected by chronic wounds by 2026 [32]. These wounds, including diabetic foot ulcers, venous leg ulcers, and pressure ulcers, persist due to factors such as chronic inflammation, infection, vascular insufficiency, and impaired cellular responses [32]. The mortality rate over 5 years for chronic wounds appears to be higher than for certain prevalent types of cancer, underscoring the urgent need for more effective therapeutic interventions [32].
Traditional wound care methods often fail to address the complex microenvironment of chronic wounds. In response, advanced wound dressings, particularly hydrogels, have gained considerable attention for their proficiency in establishing ideal conditions for wound healing [32]. Hydrogels are three-dimensional, hydrophilic polymeric networks capable of absorbing and retaining large quantities of water or biological fluids while maintaining structural integrity [33]. Their unique properties—high porosity, biocompatibility, tunable degradation, ability to maintain a moist wound environment, and structural resemblance to the native extracellular matrix (ECM)—create favorable conditions for cellular migration and proliferation [32] [33].
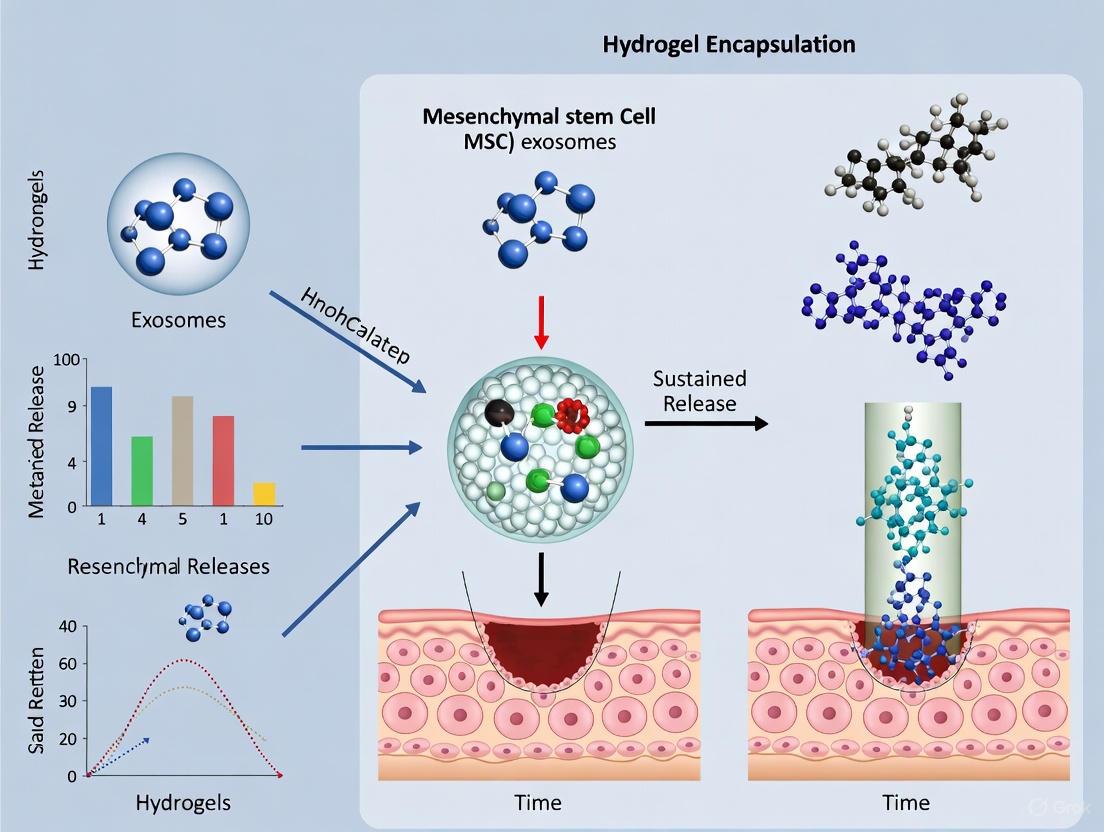
The emergence of "active dressings" represents a significant advancement in wound care. These innovative systems are created by infusing hydrogels with bioactive molecules such as antibiotics, stem cells, anti-inflammatory agents, antioxidants, and growth factors to actively accelerate the healing process [32]. Particularly promising is the encapsulation of mesenchymal stem cell (MSC)-derived exosomes within hydrogels, which combines the regenerative capabilities of exosomes with the sustained delivery and protective environment provided by the hydrogel matrix [3] [34]. This approach addresses critical limitations of conventional therapies and opens new avenues for targeted, effective wound management.
Material Foundations: Natural vs. Synthetic Hydrogels
Hydrogels can be systematically classified based on their origin into natural, synthetic, and hybrid categories. Each class offers distinct advantages and limitations for wound healing applications, as summarized in Table 1.
Table 1: Comparative Analysis of Natural and Synthetic Hydrogels for Wound Healing
| Property | Natural Hydrogels | Synthetic Hydrogels |
|---|---|---|
| Biocompatibility | Inherently high due to biological origin [35] | Must be engineered; can achieve excellent biocompatibility [33] |
| Biodegradability | Enzymatically degraded; products are naturally metabolized [35] | Degradation must be designed into polymer structure [33] |
| Mechanical Strength | Generally poor and variable between batches [33] | Highly tunable and reproducible [33] |
| Bioactive Signals | Intrinsic cell-adhesive motifs and bioactivity [35] | Typically bio-inert unless functionalized [33] |
| Batch-to-Batch Variability | High due to natural sourcing [33] | Very low due to controlled synthesis [33] |
| Degradation Control | Difficult to control precisely [33] | Highly controllable via crosslinking density and chemistry [33] |
| Cost | Generally lower cost [32] | Can be more expensive [32] |
| Representative Polymers | Alginate, Chitosan, Gelatin, Hyaluronic Acid, Collagen [32] [35] | Poly(ethylene glycol) (PEG), Poly(vinyl alcohol) (PVA), Poly(acrylic acid) (PAA) [32] [36] |
Natural Hydrogels
Natural hydrogels are derived from biological sources such as polysaccharides and proteins. Their foremost advantage is their inherent biocompatibility, biodegradability, and presence of native bioactive motifs that support cellular adhesion and proliferation [35].
- Alginate: Derived from brown seaweed, alginate forms gentle gels in the presence of divalent cations like calcium. It is highly absorbent, creating a moist wound environment, but has limited mechanical strength and cell adhesion sites unless modified [32] [35].
- Chitosan: A polysaccharide obtained from chitin in crustacean shells, chitosan is notably biocompatible, biodegradable, and possesses inherent antimicrobial properties, making it particularly valuable for infected wounds [32] [35].
- Gelatin: Derived from denatured collagen, gelatin contains arginine-glycine-aspartic acid (RGD) sequences that promote cell adhesion and migration. It is a key component of many hybrid systems and is widely used in biofabrication [36] [37].
- Hyaluronic Acid (HA): A major component of the native ECM, HA plays crucial roles in inflammation and tissue regeneration. HA-based hydrogels can be modified to create scaffolds that guide the wound healing process [37].
Synthetic Hydrogels
Synthetic hydrogels are produced from man-made polymers, offering precise control over their physical and chemical properties, including mechanical strength, degradation rate, and water content [33].
- Poly(ethylene glycol) (PEG): PEG is a neutral, hydrophilic polymer known for its "stealth" properties and resistance to protein adsorption. It is highly tunable through various crosslinking mechanisms, including photopolymerization, but requires functionalization with adhesive ligands to support cell attachment [33] [37].
- Poly(vinyl alcohol) (PVA): PVA can form hydrogels through physical crosslinking (e.g., freeze-thaw cycles) or chemical crosslinking. It is known for its high water content, elasticity, and ability to form stable matrices, as demonstrated in its use with gelatin and borax to create self-healing dressings [36].
Hybrid and "Smart" Hydrogels
Hybrid hydrogels combine natural and synthetic polymers to harness the advantages of both material classes [32] [33]. A prominent example is gelatin-methacrylate (GelMA), which integrates the bioactivity of gelatin with the controllable photopolymerization of synthetic chemistry [33].
Furthermore, "smart" or stimuli-responsive hydrogels that react to environmental cues such as pH, temperature, enzymes, or light are at the forefront of wound care innovation [33]. These systems can provide on-demand drug release or adapt their properties in response to the dynamic wound microenvironment.
Advanced Application: Hydrogel Encapsulation of MSC Exosomes
Rationale for Exosome Encapsulation
Mesenchymal stem cell (MSC)-derived exosomes have emerged as a powerful cell-free therapeutic paradigm for regenerative medicine. These nano-sized extracellular vesicles (30-150 nm) act as intercellular communicators, transferring a cargo of proteins, lipids, and nucleic acids (e.g., microRNAs) that can modulate inflammation, promote angiogenesis, inhibit apoptosis, and enhance cell migration and proliferation [3] [34]. Compared to whole MSC therapy, exosomes offer significant advantages: they lack tumorigenic potential, do not self-replicate, present a lower risk of immune rejection, and are easier to store and handle [3] [34].
However, the therapeutic efficacy of free exosomes is limited by their rapid clearance from the body and short half-life at the injury site [3] [34]. Hydrogel encapsulation provides a strategic solution to these challenges by serving as a protective reservoir that localizes the exosomes and enables their sustained, controlled release at the wound site [3] [37]. This synergistic combination enhances retention and bioavailability, thereby potentiating the therapeutic effect.
Exosome-Laden Hydrogel System Design
The design of an effective exosome-laden hydrogel dressing requires careful consideration of multiple components and their interactions. The following diagram illustrates the key functional layers and their roles in promoting wound healing.
Protocol: Fabrication and Characterization of an Exosome-Laden Injectable Hydrogel
This protocol details the development of an injectable hyaluronic acid-based hydrogel loaded with MSC-derived exosomes for chronic wound healing, based on established methodologies [3] [9] [34].
Part A: Isolation and Characterization of MSC-Derived Exosomes
Cell Culture and Conditioning:
- Culture human MSCs (e.g., from adipose tissue or bone marrow) in standard growth medium to 70-80% confluence.
- Replace growth medium with a serum-free, exosome-depleted conditioning medium.
- Incubate for 24-48 hours. Collect the conditioned medium.
Exosome Isolation (Differential Ultracentrifugation):
- Centrifuge the conditioned medium at 300 × g for 10 minutes to remove cells.
- Transfer supernatant and centrifuge at 2,000 × g for 20 minutes to remove dead cells.
- Transfer supernatant and centrifuge at 10,000 × g for 30 minutes to remove cell debris.
- Filter the supernatant through a 0.22 µm filter.
- Ultracentrifuge the filtrate at 100,000 × g for 70 minutes at 4°C.
- Discard supernatant and resuscent the exosome pellet in sterile phosphate-buffered saline (PBS).
- Repeat the ultracentrifugation wash step.
- Resuspend the final, purified exosome pellet in a small volume of PBS (e.g., 100-200 µL).
Exosome Characterization:
- Nanoparticle Tracking Analysis (NTA): Dilute exosome sample in PBS and inject into the NTA system to determine particle size distribution and concentration.
- Transmission Electron Microscopy (TEM): Adhere exosomes to a Formvar-carbon coated grid, stain with 1-2% uranyl acetate, and image to confirm cup-shaped morphology.
- Western Blotting: Lyse exosomes and probe for positive markers (e.g., CD63, CD81, TSG101) and negative markers (e.g., Calnexin).
Part B: Fabrication of the Injectable Hydrogel
Hydrogel Precursor Solution:
- Dissolve thiolated hyaluronic acid (HA-SH) and maleimide-functionalized PEG (PEG-MAL) in a chilled, degassed PBS solution (pH 7.4) at a predetermined stoichiometric ratio (e.g., 1:1 thiol:maleimide) to a final polymer concentration of 2-4% w/v.
- Gently mix the two precursor solutions on ice to slow the gelation kinetics.
Exosome Loading:
- Immediately after mixing the polymer precursors, add the concentrated exosome suspension (from Part A) to the solution and mix gently by pipetting to ensure homogeneous distribution. A typical loading concentration is 1-5 × 10^10 exosome particles per mL of hydrogel precursor.
- Note: Avoid vigorous mixing or vortexing to preserve exosome integrity.
In Situ Crosslinking and Application:
- Draw the exosome-loaded precursor solution into a sterile syringe.
- The solution remains injectable for several minutes at room temperature.
- Apply the solution directly onto the wound bed. The hydrogel will form in situ within minutes via a rapid, cytocompatible Michael-type addition reaction between the thiol and maleimide groups.
Part C: In Vitro Functional Assays
Exosome Release Kinetics:
- Incubate pre-formed hydrogel discs in PBS at 37°C under gentle agitation.
- At predetermined time points, collect the release medium and replace it with fresh PBS.
- Quantify released exosomes using NTA or a protein assay (e.g., BCA). Plot cumulative release over time.
Bioactivity Assessment:
- Use the conditioned release medium from the assay above.
- Apply to in vitro models, such as:
- Human Dermal Fibroblast (HDF) Migration Scratch Assay.
- Human Umbilical Vein Endothelial Cell (HUVEC) Tube Formation Assay to assess pro-angiogenic potential.
- Compare results against controls (free exosomes, blank hydrogel release medium).
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Research Reagents for Developing Exosome-Laden Hydrogels
| Reagent / Material | Function / Role | Example & Notes |
|---|---|---|
| Mesenchymal Stem Cells (MSCs) | Source of therapeutic exosomes. | Human ADSCs or BM-MSCs. Characterize per ISCT guidelines (CD105+, CD73+, CD90+, CD45-, CD34-, CD14-) [3] [37]. |
| Thiolated Hyaluronic Acid (HA-SH) | Natural polymer component of the injectable hydrogel matrix. Provides biocompatibility and bioactivity. | Functionalized with thiol (-SH) groups for crosslinking. Commercially available or synthesized in-lab. |
| PEG-Maleimide (PEG-MAL) | Synthetic polymer component. Forms the crosslinked network with HA-SH. | Typically 4-arm PEG-MAL (MW ~20kDa) is used. Provides controlled mechanical properties. |
| Ultracentrifuge | Essential equipment for isolating exosomes from conditioned media. | Critical for obtaining a pure exosome preparation for loading. |
| Nanoparticle Tracking Analyzer (NTA) | Key instrument for characterizing exosome size, distribution, and concentration. | Also used to track exosome release from hydrogels in release studies. |
| Fluorescent Cell Tracker Dyes (e.g., Dio, Dil) | Used to label exosomes for visualization and tracking in cellular uptake studies. | Confirms internalization of released exosomes by target cells (e.g., fibroblasts) [36]. |
Signaling Pathways and Therapeutic Mechanisms
The therapeutic efficacy of MSC exosomes in wound healing is mediated through the delivery of their cargo, which modulates key cellular processes and signaling pathways. The following diagram summarizes the primary mechanisms through which exosome-loaded hydrogels promote healing.
As illustrated, exosomes derived from different MSC sources carry distinct cargo profiles:
- Adipose-Derived Stem Cell (ADSC) Exosomes: Rich in miRNAs like miR-125a and miR-31 that promote angiogenesis, and miR-192-5p which can regulate scarring by targeting IL-17RA [3].
- Bone Marrow MSC (BMSC) Exosomes: Contain miRNAs such as miR-23a-3p that promote the polarization of pro-inflammatory M1 macrophages to pro-healing M2 macrophages, reducing early inflammatory responses [3].
Hydrogels, as versatile biomaterial platforms, have evolved from simple moisture-providing dressings to sophisticated, bioactive systems capable of directing the complex process of wound repair. The integration of MSC-derived exosomes into natural, synthetic, and hybrid hydrogels represents a paradigm shift towards targeted, sustained, and highly effective therapy for chronic wounds. This approach successfully addresses the limitations of both standalone exosome therapy and passive wound dressings.
Future directions in this field are poised to integrate cutting-edge technologies. 4D bioprinting will allow the fabrication of dynamic hydrogel scaffolds that can change shape or function over time in response to wound cues [33]. Furthermore, Artificial Intelligence (AI) is set to revolutionize hydrogel design and wound monitoring. AI-driven models can predict optimal hydrogel formulations for specific wound types and analyze real-time data from sensors integrated into conductive hydrogels to predict healing trajectories and optimize treatment plans [38]. The convergence of advanced materials biology, and engineering intelligence promises a new era of personalized, predictive, and highly effective wound management.
The encapsulation of mesenchymal stem cell (MSC)-derived exosomes into hydrogel networks represents a paradigm shift in regenerative medicine, particularly for sustained therapeutic release in wound healing applications. This approach synergistically combines the bioactive properties of exosomes—such as their ability to modulate inflammation, promote angiogenesis, and facilitate cell proliferation—with the structural and controlled-release advantages of hydrogel biomaterials. Exosomes are nano-sized extracellular vesicles (typically 30-150 nm) that facilitate intercellular communication by transferring proteins, lipids, and nucleic acids to recipient cells [1] [16]. However, their therapeutic application faces significant challenges, including rapid clearance from the body, limited retention at injury sites, and susceptibility to degradation in the harsh microenvironment of chronic wounds [1] [39]. Hydrogel-based delivery systems address these limitations by providing a protective, three-dimensional network that can sustain exosome release and maintain their bioactivity at the wound site.
The strategic integration of exosomes into hydrogels is especially crucial for diabetic wound management, where the healing process is often compromised by persistent inflammation, impaired angiogenesis, and oxidative stress [17] [39]. Conventional wound dressings and standalone exosome therapies have demonstrated suboptimal efficacy in this challenging context, necessitating advanced delivery platforms that can provide spatiotemporal control over therapeutic release [17]. By harnessing various encapsulation techniques, researchers can tailor hydrogel-exosome composites to meet the specific requirements of different wound types and healing stages, ultimately accelerating closure and promoting complete skin regeneration with functional appendages [39] [19].
Core Encapsulation Strategies and Mechanisms
Physical Adsorption and Entrapment
Physical adsorption represents one of the simplest approaches for loading exosomes into hydrogel networks. This technique leverages non-covalent interactions—including electrostatic forces, hydrogen bonding, and van der Waals interactions—between exosomal surfaces and the hydrogel polymer chains [17]. The negatively charged surfaces of many exosomes facilitate their adsorption to cationic polymers within hydrogels, such as the ε-poly-L-lysine (EPL) component in F127/OHA-EPL (FHE) hydrogel systems [39]. This mechanism allows for spontaneous exosome incorporation during hydrogel formation without requiring chemical modification that might compromise exosome integrity.
The release kinetics in physically adsorbed systems are primarily governed by hydrogel swelling dynamics and the dissociation of non-covalent bonds. Research demonstrates that this approach can be enhanced by designing hydrogels with tunable degradation profiles, where exosome release correlates with hydrogel matrix breakdown [17] [40]. For instance, in recombinant human collagen methacrylate (RHCMA) hydrogels, exosome release occurs progressively as the hydrogel swells and degrades, with approximately 56% of loaded exosomes released within the first 12 hours and over 90% released after 48 hours [19]. While this method offers simplicity and preserves exosome bioactivity, it may result in relatively rapid initial release bursts, making it particularly suitable for applications requiring immediate therapeutic action followed by sustained delivery.
In Situ Hybrid Cross-Linking
In situ hybrid cross-linking represents a more integrated approach where exosomes are incorporated during the hydrogel formation process. This strategy typically involves mixing exosomes with hydrogel precursors before cross-linking is initiated, resulting in exosomes being entrapped within the evolving polymer network [17]. Various cross-linking mechanisms can be employed, including photoinitiation (e.g., using gelatin methacryloyl (GelMA) systems) [41], chemical cross-linking (e.g., Schiff base formation between oxidized hyaluronic acid and ε-poly-L-lysine) [39], and enzymatic cross-linking.
The key advantage of this technique is the uniform distribution of exosomes throughout the hydrogel matrix and enhanced retention due to physical confinement within the cross-linked network. Studies on GelMA hydrogels have demonstrated that this approach significantly prolongs exosome retention at wound sites compared to direct exosome application [41]. Additionally, the cross-linking density can be precisely tuned to control exosome release kinetics—higher cross-linking densities generally result in slower release profiles due to reduced mesh sizes within the hydrogel network [40]. This method is particularly valuable for creating injectable hydrogel systems that can conform to irregular wound geometries and provide localized exosome delivery.
Covalent Conjugation and Post-Preloading Cross-Linking
For applications requiring even more sustained release profiles, covalent conjugation strategies have been developed to tether exosomes directly to the hydrogel network. This approach involves forming stable chemical bonds between functional groups on exosomal surfaces (e.g., amine, carboxyl, or thiol groups) and complementary reactive groups within the hydrogel matrix [17]. Although less common due to concerns about potentially compromising exosome bioactivity, advanced techniques have been developed that selectively target surface molecules without affecting internal cargo.
Post-preloading cross-linking represents a hybrid approach where exosomes are first loaded into a pre-formed hydrogel, followed by additional cross-linking steps to enhance retention. This method creates a more densely cross-linked periphery around the entrapped exosomes, effectively reducing their diffusion rate from the hydrogel [17]. The resulting sustained release profiles are particularly advantageous for chronic wound healing, where prolonged therapeutic presence is required to modulate the prolonged inflammatory phase and support tissue regeneration over several weeks [39]. Additionally, some covalently conjugated systems can be engineered to respond to specific microenvironmental cues in chronic wounds, such as pH changes or elevated enzyme levels, enabling smart release triggered by the wound state itself.
Table 1: Comparison of Major Exosome Encapsulation Strategies
| Encapsulation Strategy | Mechanism of Integration | Release Kinetics | Advantages | Limitations |
|---|---|---|---|---|
| Physical Adsorption | Non-covalent interactions (electrostatic, hydrogen bonding) | Relatively rapid initial release followed by sustained phase | Simple procedure, maintains exosome bioactivity, minimal chemical modification | Potential burst release, moderate retention efficiency |
| In Situ Hybrid Cross-Linking | Entrapment during hydrogel formation | Controlled release dependent on cross-linking density | Uniform distribution, conformal coverage of wound bed, tunable release kinetics | Potential exposure to cross-linking agents, optimization required for each system |
| Covalent Conjugation | Stable chemical bonds between exosomes and hydrogel matrix | Sustained, prolonged release over extended periods | Enhanced retention, protection from degradation, suitable for chronic applications | Risk of modifying critical surface molecules, complex chemistry required |
| Post-Preloading Cross-Linking | Secondary cross-linking after exosome loading | Extended release profiles | Combines benefits of physical entrapment with enhanced retention, reduced initial burst | Additional processing steps, potential for heterogeneous distribution |
Performance Metrics and Functional Outcomes
Quantitative Analysis of Release Kinetics and Wound Closure
The therapeutic efficacy of exosome-laden hydrogels is highly dependent on their release kinetics, which directly influence wound healing outcomes. Systematic evaluation of various hydrogel systems has yielded crucial quantitative data linking encapsulation strategies to functional performance. In diabetic wound models, sustained exosome release from hydrogels has demonstrated statistically significant improvements in healing parameters compared to both control treatments and bolus exosome delivery.
Research on F127/OHA-EPL (FHE) hydrogels has revealed their ability to provide pH-responsive exosome release, with approximately 80% cumulative release under physiological conditions over 7 days [39]. This sustained delivery translated to enhanced wound healing outcomes, with FHE@exo hydrogels achieving nearly 95% wound closure within 14 days in diabetic rat models—significantly higher than the approximately 70% closure observed in exosome-only treatment groups. Similarly, studies on recombinant human collagen methacrylate (RHCMA) hydrogels demonstrated that 10% RHCMA concentration provided optimal release kinetics, with 56.27 ± 4.48% of loaded ucMSC-exos released within the first 12 hours and 92.27 ± 3.19% cumulative release after 48 hours [19]. These release profiles directly correlated with accelerated wound closure, underscoring the importance of matching release kinetics to the biological timeline of wound healing processes.
Table 2: Performance Metrics of Exosome-Laden Hydrogel Systems
| Hydrogel System | Exosome Source | Cumulative Release Profile | Wound Closure Rate | Key Therapeutic Effects |
|---|---|---|---|---|
| F127/OHA-EPL (FHE) | Adipose MSC-derived | ~80% over 7 days (pH-responsive) | ~95% closure in 14 days (diabetic rat model) | Enhanced angiogenesis, re-epithelization, collagen deposition |
| RHCMA (10%) | Umbilical cord MSC-derived | 56.27±4.48% in 12h; 92.27±3.19% in 48h | Significantly accelerated vs. controls | Reduced inflammation, promoted angiogenesis and collagen formation |
| PVA/Gelatin-Borax | Adipose MSC-derived (exosome-coated oxygen nanobubbles) | Sustained release over 72 hours | Enhanced healing in full-thickness rat wound model | Ameliorated hypoxia, reduced inflammation, promoted angiogenesis |
| GelMA Hydrogel | Human umbilical MSC-derived | Controlled release over 5-7 days | Promoted wound closure and re-epithelization | Transferred mitochondrial proteins, enhanced fibroblast proliferation |
Functional Mechanisms and Pathway Modulation
The therapeutic benefits of exosome-laden hydrogels extend beyond simple physical wound closure to complex modulation of cellular signaling pathways critical for coordinated tissue regeneration. MSC-derived exosomes contain diverse bioactive cargo—including microRNAs, proteins, and lipids—that orchestrate multiple aspects of the healing process through distinct mechanistic pathways.
Angiogenesis, essential for delivering oxygen and nutrients to healing tissues, is significantly enhanced by exosome-laden hydrogels through the transfer of pro-angiogenic miRNAs and proteins. Specifically, exosomes derived from adipose-derived stem cells (ADSCs) are rich in miR-125a and miR-31, which can be transferred to vascular endothelial cells to stimulate proliferation and promote new blood vessel formation [16]. Similarly, bone marrow MSC-derived exosomes have been shown to activate the Ras/Erk pathway, leading to significant angiogenesis, proliferation, and migration [16]. These processes are further supported by the sustained presentation of vascular endothelial growth factor (VEGF) and other growth factors delivered via controlled release from hydrogel platforms [17].
Inflammation resolution represents another critical mechanism through which exosome-laden hydrogels promote healing. In chronic wounds, prolonged inflammation prevents progression to proliferative phases of healing. MSC-derived exosomes address this by promoting macrophage polarization from pro-inflammatory M1 phenotypes to anti-inflammatory M2 phenotypes [16]. For instance, bone marrow MSC-derived exosomes promote this transition via miR-23a-3p, reducing early inflammatory responses at wound sites [16]. This immunomodulatory effect is particularly important in diabetic wounds, where chronic inflammation significantly impedes healing.
Additionally, exosome-laden hydrogels directly influence extracellular matrix (ECM) remodeling through the regulation of fibroblast function and collagen deposition. Studies demonstrate that umbilical cord MSC-derived exosomes loaded in RHCMA hydrogels significantly enhance collagen formation and organization in wound beds [19]. This improved ECM deposition provides better structural support for regenerating tissues and contributes to the restoration of skin barrier function. The combined modulation of these multiple pathways—angiogenesis, inflammation resolution, and ECM remodeling—underscores the multifaceted therapeutic mechanism of exosome-laden hydrogel systems in wound healing applications.
Experimental Protocols for Hydrogel-Exosome Composite Fabrication
Protocol 1: Physical Adsorption in F127/OHA-EPL (FHE) Hydrogel
This protocol details the preparation of an injectable, self-healing antibacterial FHE hydrogel loaded with adipose-derived MSC exosomes through physical adsorption mechanisms [39].
Materials Preparation:
- Prepare oxidized hyaluronic acid (OHA) by reacting hyaluronic acid (1% w/v) with sodium periodate (0.5 M) for 6 hours in the dark at room temperature. Terminate the reaction by adding ethylene glycol and dialyze against distilled water for 2 days.
- Prepare ε-poly-L-lysine (EPL) solution at 10% w/v in deionized water.
- Prepare Pluronic F127 solution at 20% w/v in phosphate-buffered saline (PBS).
- Isolate exosomes from adipose-derived MSC culture supernatant using sequential ultracentrifugation: 300 × g for 10 min, 2,000 × g for 10 min, 15,000 × g for 40 min, and 100,000 × g for 70 min twice. Resuspend the final pellet in PBS and characterize by nanoparticle tracking analysis, transmission electron microscopy, and Western blotting for CD9, CD63, and TSG101 markers.
Hydrogel Formation and Exosome Loading:
- Mix F127 solution, OHA solution, and EPL solution at a volume ratio of 2:1:1 at 4°C.
- Add exosomes to the mixture at a concentration of 50-100 μg/mL and mix gently by pipetting.
- Incubate the mixture at 37°C for 10 seconds to facilitate rapid gelation through Schiff base formation between OHA and EPL, combined with F127 thermal responsiveness.
- Characterize the mechanical properties using rheometry to confirm self-healing behavior through strain recovery tests.
Quality Control and Application:
- Assess exosome release kinetics by incubating FHE@exo hydrogel in PBS at pH 7.4 and 6.5, collecting supernatant at predetermined time points, and quantifying exosome concentration using BCA protein assay or nanoparticle tracking analysis.
- Evaluate antibacterial activity against common wound pathogens (e.g., Staphylococcus aureus, Pseudomonas aeruginosa) using zone of inhibition assays.
- For in vivo application, apply the FHE@exo hydrogel directly to wounds using a syringe, allowing it to conform to wound contours and form a protective, bioactive barrier.
Protocol 2: In Situ Encapsulation in Recombinant Human Collagen Methacrylate (RHCMA) Hydrogel
This protocol describes the fabrication of a photo-crosslinkable recombinant human collagen hydrogel for sustained delivery of umbilical cord MSC-derived exosomes [19].
Materials Preparation:
- Synthesize recombinant human collagen methacrylate (RHCMA) by reacting type I recombinant human collagen (10% w/v) with methacrylate anhydride (0.5% v/v) in PBS on ice for 24 hours. Purify by dialysis against distilled water for 3 days and lyophilize.
- Prepare photoinitiator solution (Irgacure 2959) at 1% w/v in PBS.
- Islate umbilical cord MSC-derived exosomes from conditioned media using ultracentrifugation as described in Protocol 1. Characterize exosomes by dynamic light scattering (size distribution: 103.1 ± 3.8 nm) and confirm presence of CD9, CD63, and CD81 markers.
Hydrogel Formation and Exosome Encapsulation:
- Prepare 10% w/v RHCMA pre-gel solution by dissolving in PBS containing 0.5% w/v photoinitiator.
- Mix ucMSC-exosomes with RHCMA pre-gel solution at a concentration of 1 × 10^10 particles/mL.
- Transfer the mixture to a mold matching the wound dimensions and expose to UV light (365 nm, 5 mW/cm²) for 60 seconds to initiate cross-linking.
- Sterilize the resulting ucMSC-exos@RHCMA hydrogel under UV light for 15 minutes per side before application.
Characterization and Validation:
- Determine swelling ratio by measuring weight change after immersion in PBS for 24 hours: Swelling Ratio = (Ww - Wd)/Wd, where Ww is wet weight and Wd is dry weight.
- Evaluate compressive mechanical properties using a universal testing machine with a 50 N load cell at a compression rate of 1 mm/min.
- Assess sustained release profile by incubing in PBS at 37°C and quantifying exosome release at 0, 2, 6, 12, 24, and 48 hours using microBCA assay or ELISA for specific exosomal markers.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagent Solutions for Hydrogel-Exosome Research
| Reagent/Material | Function/Application | Key Characteristics | Representative Examples |
|---|---|---|---|
| Polysaccharide Polymers | Structural backbone for hydrogel formation | Biocompatibility, tunable mechanical properties, bioactive motifs | Oxidized hyaluronic acid (OHA), alginate, chitosan [39] |
| Protein-Based Polymers | ECM-mimetic hydrogel components | Cell adhesion sites, enzymatic degradation sensitivity | Recombinant human collagen, gelatin, gelatin methacryloyl (GelMA) [19] |
| Synthetic Polymers | Controlled mechanical properties and degradation | Precise control over cross-linking and architecture | Pluronic F127, polyvinyl alcohol (PVA), polyethylene glycol (PEG) derivatives [36] [39] |
| Cross-Linking Agents | Facilitate hydrogel network formation | Determines gelation kinetics and mechanical properties | Borax (dynamic bonds), photoinitiators (Irgacure 2959), genipin [36] [19] |
| Exosome Isolation Tools | Purification of MSC-derived exosomes | Maintain exosome integrity and bioactivity | Ultracentrifugation systems, size-exclusion chromatography, polymer-based precipitation kits [1] [19] |
| Characterization Instruments | Quality assessment of exosomes and hydrogels | Multi-parameter analysis of physical and chemical properties | Nanoparticle tracking analyzer, transmission electron microscope, rheometer [39] [19] |
Visualizing Encapsulation Strategies and Experimental Workflows
Diagram 1: Experimental Workflow for Exosome Encapsulation in Hydrogel Networks. This flowchart outlines the major pathways for developing exosome-laden hydrogel systems, from strategy selection through characterization and final application.
The strategic encapsulation of MSC-derived exosomes within hydrogel networks represents a significant advancement in regenerative medicine, particularly for challenging wound healing scenarios. The encapsulation techniques detailed in this document—physical adsorption, in situ cross-linking, and covalent conjugation—provide researchers with a versatile toolkit for developing tailored therapeutic systems that address the limitations of conventional wound treatments. The quantitative data presented demonstrates unequivocally that sustained exosome delivery via hydrogels enhances key healing parameters, including angiogenesis, inflammation resolution, and extracellular matrix remodeling.
Looking forward, several emerging trends promise to further refine these technologies. The development of "smart" hydrogels that respond to specific wound microenvironment cues (e.g., pH, enzyme activity, or reactive oxygen species levels) may enable more autonomous therapeutic release aligned with healing progression [17]. Similarly, the exploration of alternative exosome sources—including comparisons between adipose-derived, bone marrow-derived, and umbilical cord-derived MSC exosomes—will help identify optimal vesicle populations for specific wound types [19]. Advances in production methodologies, such as the development of exosome mimetics with enhanced production yield and consistent cargo loading, may address current scalability challenges [41]. As these technologies mature, the integration of exosome-laden hydrogels into clinical wound care protocols appears increasingly imminent, promising new solutions for patients suffering from chronic, non-healing wounds.
In the field of regenerative medicine, hydrogel scaffolds have emerged as a foundational technology for the controlled delivery of therapeutic exosomes derived from mesenchymal stromal cells (MSCs). These exosomes, nano-sized vesicles carrying bioactive cargo such as proteins, lipids, and nucleic acids, orchestrate complex processes in tissue repair, including immunomodulation, angiogenesis, and cellular proliferation [16] [2]. However, a significant translational challenge lies in their rapid clearance from the target site when administered in their free form, which severely limits their therapeutic potential [16] [42].
Encapsulating exosomes within hydrogel scaffolds presents an elegant solution, enabling spatiotemporally controlled release that aligns with the prolonged timeline of tissue regeneration [17] [40]. The critical task for researchers is to accurately profile these release kinetics, as the rate and duration of exosome delivery are direct determinants of therapeutic efficacy. This Application Note provides a detailed framework for the quantitative analysis of sustained exosome delivery from hydrogel systems, offering standardized protocols and data interpretation guidelines essential for advancing robust, clinically viable therapeutic products.
Hydrogel-Exosome System Fundamentals
Key Components and Their Properties
The performance of a hydrogel-exosome delivery system is governed by the interplay between the scaffold's material properties and the biological characteristics of the exosomes.
Table 1: Key Components of Hydrogel-Exosome Systems
| Component | Types & Examples | Key Characteristics / Functions | Influence on Release Kinetics |
|---|---|---|---|
| Hydrogel Material | Natural Polymers: Recombinant Human Collagen (RHC), Hyaluronic Acid (HA), Chitosan, Alginate, Fibrin [42] [19] [40] | High biocompatibility, inherent biodegradability, often contain cell-adhesion motifs. Mimic native extracellular matrix (ECM). | Swelling behavior, degradation rate, and pore size determine diffusion speed and release duration. |
| Hydrogel Material | Synthetic Polymers: Poly(ethylene glycol) (PEG), Poly(vinyl alcohol) (PVA) [42] | Tunable mechanical properties, controllable and reproducible architecture, consistent degradation profiles. | Allows for precise engineering of crosslinking density and mesh size for predictable release. |
| Exosome Source | Adipose-Derived MSCs (ADSCs), Bone Marrow-Derived MSCs (BMSCs), Umbilical Cord MSCs (ucMSCs) [16] [19] [43] | Source determines cargo profile (e.g., miRNAs, proteins). ADSC-exos promote angiogenesis; BMSC-exos boost cell growth; ucMSC-exos regulate inflammation [19]. | Cargo profile influences downstream therapeutic effects but not the primary physical release of vesicles. |
| Crosslinking Method | Physical (e.g., ionic, thermal), Chemical (e.g., photo-crosslinking, enzymatic) [42] | Determines hydrogel network stability and responsiveness. Photo-crosslinked methacrylated collagen (RHCMA) allows precise control over gelation [19]. | Crosslinking density directly defines mesh size and degradation rate, which are primary controllers of sustained release. |
System Assembly and Release Workflow
The process of assembling the delivery system and analyzing its release profile follows a logical sequence, from hydrogel preparation to kinetic modeling.
Quantitative Release Kinetics Data
The release profile of exosomes from a hydrogel is a quantifiable metric that dictates therapeutic dosing. The following data, compiled from recent studies, provides a benchmark for expected performance.
Table 2: Experimental Release Kinetics from Representative Hydrogel Systems
| Hydrogel System | Exosome Source | Experimental Model | Key Release Metrics | Primary Release Mechanism |
|---|---|---|---|---|
| RHCMA (10% w/v) [19] | ucMSC-exos | In vitro, PBS, 37°C | - 56.3% released in first 12 h- 92.3% cumulative release at 48 h | Diffusion-dominated initial release, followed by sustained release as hydrogel degrades. |
| Sodium Alginate Hydrogel [44] | Decidual Stromal Cell-exos | In vitro | - 95% sustained release over 5 days | Sustained release covering critical repair phases, ideal for uterine cavity retention. |
| Hypoxia-pretreated ADSC-exo Hydrogel [17] | ADSC-exos | Rodent wound model | - ~30% increase in wound healing rate- 72-hour sustained VEGF delivery in vitro | Synergistic effect of bioactive exosome cargo and hydrogel-controlled release enhancing angiogenesis. |
Experimental Protocols
Protocol 1: Profiling In Vitro Release Kinetics
This protocol details the standard method for characterizing the release profile of exosomes from hydrogel scaffolds under simulated physiological conditions.
Materials and Reagents
- Purified Exosomes: Isolated from MSC-conditioned media via ultracentrifugation or tangential flow filtration [19] [45]. Characterize particle size (e.g., ~103 nm for MSC-exos) and concentration via Nanoparticle Tracking Analysis (NTA) [19].
- Hydrogel Pre-cursor Solution: e.g., 10% (w/v) methacrylated recombinant human collagen (RHCMA) in PBS [19].
- Release Medium: Phosphate-buffered saline (PBS, pH 7.4) or cell culture medium, optionally with 0.5% (w/v) bovine serum albumin (BSA) to prevent adhesion.
- Fluorescent Label (Optional): Lipophilic dyes (e.g., DiI, DiD) for exosome tracking [19].
- Equipment: Microplate reader, fluorescence spectrophotometer, NTA instrument, low-speed orbital shaker, 24-well cell culture plates, microcentrifuges.
Step-by-Step Procedure
Hydrogel Loading and Formation:
- Mix the purified exosomes (e.g., 1 × 10^10 particles) thoroughly with the hydrogel pre-cursor solution. For photo-crosslinkable hydrogels like RHCMA, add a photoinitiator (e.g., Irgacure 2959) [19].
- Transfer 100-200 µL of the exosome-hydrogel mixture to each well of a 24-well plate.
- Induce gelation per the hydrogel's specification (e.g., expose to UV light (365 nm, 5 mW/cm²) for 60 seconds for RHCMA) [19].
Elution and Sampling:
- Carefully add 1 mL of pre-warmed (37°C) release medium to each well, ensuring the hydrogel is fully immersed.
- Place the plate on an orbital shaker in a 37°C incubator, agitating at low speed (e.g., 60 rpm).
- At predetermined time points (e.g., 1, 2, 4, 8, 12, 24, 48, 72 hours), collect the entire release medium and replace it with an equal volume of fresh, pre-warmed medium to maintain sink conditions [19].
Exosome Quantification:
- Quantitative Method: Determine the amount of exosomes in the collected medium. For fluorescently labeled exosomes, measure fluorescence intensity and compare to a standard curve. Alternatively, use protein assays (BCA) or ELISA for specific exosomal surface markers (e.g., CD63, CD9) [19].
- Characterization Method: Use Nanoparticle Tracking Analysis (NTA) on selected samples to confirm the size distribution and integrity of the released exosomes.
Data Analysis:
- Calculate the cumulative release percentage for each time point.
- Plot cumulative release (%) versus time to generate the release profile.
- Fit the release data to mathematical models (e.g., Higuchi, Korsmeyer-Peppas) to elucidate the dominant release mechanism.
Protocol 2: Validating Exosome Bioactivity Post-Release
It is critical to confirm that the encapsulation and release process does not compromise exosome function.
Materials and Reagents
- Cell Lines: Relevant recipient cells for functional testing (e.g., human dermal fibroblasts (HDFs) for wound healing applications).
- Cell Culture Reagents: Standard medium (e.g., DMEM), fetal bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA.
- Assay Kits: Cell Counting Kit-8 (CCK-8) or MTT for proliferation; reagents for Transwell migration assay; tube formation assay kit for angiogenesis.
Step-by-Step Procedure
- Sample Preparation: Collect released exosomes from the In Vitro Release Kinetics protocol. Concentrate the samples if necessary using centrifugal filters with a 100 kDa molecular weight cut-off.
- Cell Proliferation Assay:
- Seed HDFs in a 96-well plate (e.g., 5 × 10^3 cells/well). After 24 hours, treat the cells with fresh medium containing released exosomes, pristine exosomes (positive control), or medium alone (negative control).
- After 48-72 hours, add CCK-8 reagent and incubate for 2-4 hours. Measure the absorbance at 450 nm. Compare the results to assess if the released exosomes retain their ability to promote proliferation [19].
- Cell Migration Assay (Scratch/Wound Healing Assay):
- Seed HDFs in a 24-well plate to form a confluent monolayer. Create a scratch using a sterile pipette tip.
- Wash away debris and add medium containing released exosomes or controls.
- Image the scratch at 0, 12, and 24 hours. Quantify the percentage of wound closure. Functional exosomes will significantly enhance cell migration compared to the control [19].
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Hydrogel-Exosome Release Studies
| Item | Function / Application | Example Specifications / Notes |
|---|---|---|
| Recombinant Human Collagen (RHC) [19] | Biocompatible, tunable natural hydrogel scaffold. Avoids immunogenicity of animal-derived collagen. | Modify with methacrylate anhydride (MA) to create photo-crosslinkable RHCMA. A 10% (w/v) concentration is often optimal [19]. |
| Mesenchymal Stem Cells (MSCs) [16] [19] | Source of therapeutic exosomes. Choice of tissue source (UC, BM, AD) tailors the exosomal cargo and therapeutic effect. | Isolate from human umbilical cord (ucMSCs), bone marrow (BMSCs), or adipose tissue (ADSCs). Culture in standard media to collect conditioned medium for exosome isolation. |
| Ultracentrifugation System [19] [45] | The gold-standard method for isolating and purifying exosomes from cell culture supernatant. | Sequential centrifugation steps: 300 × g (cell removal), 2,000 × g (apoptotic bodies), 10,000 × g (microvesicles), and 100,000 × g (exosome pellet) [45]. |
| Nanoparticle Tracking Analyzer (NTA) [19] | Critical for characterizing exosome preparation and release samples. Measures particle size distribution and concentration. | Confirm exosome size is ~100-150 nm. Use to quantify the number of particles released into the medium at different time points. |
| Lipophilic Tracers (DiI, DiD) [19] | Fluorescent dyes for labeling the lipid bilayer of exosomes, enabling facile and sensitive quantification of release kinetics. | Incubate with exosomes prior to hydrogel loading. Use fluorescence plate reader for quantification in release medium. |
| Photoinitiator (Irgacure 2959) [19] | Initiates radical polymerization for crosslinking methacrylated hydrogels (e.g., GelMA, RHCMA) upon UV exposure. | Use at a low concentration (e.g., 0.05-0.1% w/v) to ensure cytocompatibility and effective crosslinking under mild UV (365 nm). |
Data Analysis and Kinetic Modeling
Interpreting release data through mathematical models is essential for understanding the underlying mechanisms and predicting in vivo performance.
Mathematical Models for Release Kinetics
Higuchi Model: ( Mt / M\infty = k_H \cdot t^{1/2} )
- Applicable for diffusion-controlled release from a planar matrix, often describes the initial release phase (<60% release) [19].
Korsmeyer-Peppas Model (Power Law): ( Mt / M\infty = k \cdot t^n )
- A versatile model used to analyze the first 60% of the release curve. The release exponent ( n ) indicates the underlying mechanism:
- ( n = 0.45 ): Fickian diffusion (Case I transport).
- ( 0.45 < n < 0.89 ): Anomalous transport (coupling of diffusion and polymer relaxation).
- ( n = 0.89 ): Case II transport (zero-order, relaxation-controlled) [19].
- A versatile model used to analyze the first 60% of the release curve. The release exponent ( n ) indicates the underlying mechanism:
Fitting the experimental data from Table 2 to these models allows researchers to determine whether release is primarily driven by simple diffusion of exosomes through the hydrogel pores or by the swelling/erosion of the hydrogel network itself.
Release Mechanisms and Functional Impact
The design of the hydrogel system directly dictates the release mechanism, which in turn influences the therapeutic outcome, as shown in the following conceptual pathway.
The meticulous profiling of exosome release kinetics from hydrogel scaffolds is not merely a quality control step but a cornerstone of developing effective regenerative therapies. The protocols and analytical frameworks outlined in this document provide a standardized approach to quantify and model this critical process. By linking specific hydrogel properties—such as polymer concentration, crosslinking density, and degradation rate—to distinct release profiles and mechanisms, researchers can move from empirical formulations to rationally designed delivery systems. This enables the precise temporal control over the presentation of therapeutic exosomes necessary to effectively guide the complex, multi-stage process of tissue repair and regeneration.
The encapsulation of mesenchymal stem cell (MSC)-derived exosomes within hydrogels represents a promising strategy for enhancing wound healing through sustained release of therapeutic cargo. This application note provides detailed protocols and methodologies for the in vitro validation of these constructs, specifically assessing their effects on three critical processes: cell migration, proliferation, and angiogenesis. These assays are essential for establishing bioactivity and optimizing therapeutic formulations before advancing to in vivo studies. The combination of exosomes and hydrogels addresses key challenges in regenerative medicine, including short exosome retention times and the need for controlled release kinetics at the wound site [3].
Comparative Performance of MSC-Exosome Hydrogels
Table 1: In vitro performance of different MSC-exosome loaded hydrogels
| Exosome Source | Hydrogel Carrier | Cell Migration Enhancement | Proliferation Effect | Angiogenic Potential | Key Bioactive Factors |
|---|---|---|---|---|---|
| Umbilical Cord MSC | Recombinant Human Collagen (RHCMA) | Significant improvement [19] | Enhanced cell growth [19] | Strong pro-angiogenic effect [19] | miRNAs, TGF-β, IL-10 [19] |
| Adipose-Derived MSC (ADSC) | Recombinant Human Collagen (RHCMA) | Improved migration [19] | Promoted proliferation [19] | Supports vessel formation [19] | VEGF, HGF, TGF-β [3] |
| Bone Marrow MSC (BMSC) | Recombinant Human Collagen (RHCMA) | Moderate enhancement [19] | Boosted cell growth/survival [19] | Moderate angiogenic effect [19] | TSG-6, TNF-α, miRNAs [3] |
| Dental Pulp MSC | Not specified | Significantly enhanced endothelial cell migration [46] | Data not provided | Strong angiogenic potential [46] | VEGF, PDGF, bFGF [46] |
Table 2: Hydrogel properties and release characteristics
| Hydrogel Type | Composition | Compressive Stress | Swelling Ratio | Exosome Release Profile | Biocompatibility |
|---|---|---|---|---|---|
| 10% RHCMA | Recombinant human collagen with methacrylate anhydride | 47.9 ± 6.6 kPa [19] | 14.5 ± 0.6 [19] | 56.27% at 12h; 92.27% at 48h [19] | Excellent, supports normal cell growth [19] |
| 17.5% RHCMA | Recombinant human collagen with methacrylate anhydride | 136.8 ± 9.6 kPa [19] | 7.3 ± 1.0 [19] | Slower release rate [19] | Excellent, supports normal cell growth [19] |
| MEMC-Gel | GelMA and dopamine with MSC/MC exosomes | Favorable mechanical properties [47] | Good absorbency [47] | Sustained release [47] | Excellent biocompatibility [47] |
Experimental Protocols
Scratch Wound Healing Assay for Cell Migration
Purpose: To evaluate the effect of MSC-exosome hydrogels on endothelial cell migration, a critical process in wound healing and angiogenesis [46].
Materials:
- Human endothelial cells (e.g., HUVECs)
- MSC-exosome loaded hydrogel (e.g., RHCMA, MEMC-Gel)
- Complete growth medium
- 12-well or 24-well cell culture plates
- Sterile pipette tips (200 μL) or cell scratcher
- Phase-contrast microscope with imaging system
- Image analysis software (e.g., ImageJ)
Methodology:
- Culture human endothelial cells in complete growth medium until they form a confluent monolayer in 12-well plates [46].
- Create a uniform "wound" or scratch in the cell monolayer using a sterile 200 μL pipette tip or specialized cell scratcher [46].
- Gently wash cells with PBS to remove detached cells and debris.
- Apply experimental conditions:
- Test group: MSC-exosome loaded hydrogel extract or direct hydrogel placement
- Control group: Standard growth medium alone
- Capture images of the scratch area immediately after wounding (0 hour) and at regular intervals (e.g., 24h, 48h) using a phase-contrast microscope [46].
- Quantify cell migration by measuring the reduction in scratch area over time using image analysis software.
- Calculate percentage wound closure using the formula: [(Initial area - Final area) / Initial area] × 100.
Key Considerations:
- Maintain consistent scratch width and imaging positions across all experimental groups.
- Perform experiments in triplicate to ensure statistical significance.
- Include serum-free or low-serum conditions to minimize proliferation-dependent migration.
Cell Proliferation Assay (CCK-8)
Purpose: To assess the proliferative effects of MSC-exosome hydrogels on target cells relevant to wound healing.
Materials:
- Fibroblasts (e.g., 3T3 cells) or endothelial cells
- MSC-exosome loaded hydrogel extracts
- Cell Counting Kit-8 (CCK-8)
- 96-well cell culture plates
- Microplate reader
- Incubator (37°C, 5% CO₂)
Methodology:
- Seed cells in 96-well plates at an optimal density (e.g., 5 × 10³ cells/well) and incubate for 24 hours to allow attachment [19].
- Prepare hydrogel extracts by incubating MSC-exosome loaded hydrogels in culture medium for 24 hours.
- Replace standard culture medium with hydrogel extracts or control media.
- Incubate cells for predetermined time points (e.g., 24h, 48h, 72h).
- At each time point, add 10 μL of CCK-8 solution to each well and incubate for 2-4 hours at 37°C [47].
- Measure absorbance at 450 nm using a microplate reader.
- Calculate cell viability percentage relative to control groups.
Key Considerations:
- Include blank wells (medium without cells) for background subtraction.
- Ensure consistent cell seeding density across all experimental groups.
- Use multiple time points to establish proliferation kinetics.
In Vitro Angiogenesis Assay
Purpose: To evaluate the pro-angiogenic potential of MSC-exosome hydrogels through endothelial tube formation analysis.
Materials:
- Human endothelial cells (e.g., HUVECs)
- MSC-exosome loaded hydrogel extracts
- Matrigel matrix
- 96-well plates
- Tissue culture incubator (37°C, 5% CO₂)
- Phase-contrast microscope with imaging system
- Image analysis software
Methodology:
- Thaw Matrigel matrix on ice overnight at 4°C.
- Coat 96-well plates with 50-100 μL of Matrigel per well and incubate at 37°C for 30-60 minutes to allow polymerization [47].
- Harvest endothelial cells and resuspend in MSC-exosome hydrogel extracts or control media.
- Seed 2 × 10⁴ cells per well onto the polymerized Matrigel.
- Incubate cells at 37°C with 5% CO₂ for 4-16 hours.
- Capture images of tube formation using a phase-contrast microscope.
- Quantify angiogenic parameters using image analysis software:
- Total tube length
- Number of branching points
- Number of complete loops
Key Considerations:
- Use early passage endothelial cells for optimal tube formation capacity.
- Maintain consistent Matrigel lot between experiments due to batch variability.
- Image multiple fields per well to ensure representative sampling.
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential research reagents and materials
| Reagent/Material | Function/Application | Examples/Specifications |
|---|---|---|
| Recombinant Human Collagen (RHC) | Base material for biocompatible hydrogels [19] | Modified with methacrylate anhydride (RHCMA) for tunable properties [19] |
| Methacrylated Gelatin (GelMA) | Photopolymerizable hydrogel substrate [47] | Combined with dopamine for enhanced adhesion (MEMC-Gel) [47] |
| MSC-Exosomes (ucMSC, ADSC, BMSC) | Therapeutic cargo with regenerative properties [19] [3] | Isolated via ultracentrifugation; 30-150 nm diameter [19] [3] |
| Cell Counting Kit-8 (CCK-8) | Colorimetric assay for cell proliferation/viability [47] | Non-radioactive alternative to MTT assays [47] |
| Matrigel Matrix | Basement membrane extract for tube formation assays [47] | Provides substrate for endothelial network formation [47] |
| Lithium Phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) | Photoinitiator for hydrogel crosslinking [47] | Enables UV-mediated polymerization of GelMA/RHCMA [47] [19] |
| PKH26/PKH67 | Fluorescent cell membrane dyes for exosome tracking [47] | Used to label and visualize exosome uptake and distribution [47] |
Experimental Workflow and Signaling Pathways
Experimental Workflow for In Vitro Validation
Signaling Pathways in MSC-Exosome Mediated Wound Healing
Enhancing Efficacy: Overcoming Challenges in Hydrogel-Exosome Formulation
Extracellular vesicles, particularly exosomes derived from mesenchymal stem cells (MSCs), have emerged as a promising cell-free therapeutic strategy for enhancing wound healing. These nanoscale lipid bilayer vesicles (30-150 nm in diameter) serve as natural carriers of bioactive molecules—including proteins, lipids, mRNAs, and miRNAs—and mediate regenerative processes such as alleviating inflammation, promoting vascularization, and facilitating cellular proliferation and migration [48] [24]. Despite their therapeutic potential, the clinical application of free exosomes faces significant pharmacological challenges. The commonly used method of administration via injection may induce secondary damage to the skin and result in the substantial loss and waste of exosomes [49]. Enzymes present at the wound site rapidly degrade exosomes, compromising their biological activity, while the fast clearance rate from the administration site prevents the maintenance of a stable and effective concentration for an extended period [49] [50]. Without an effective delivery vehicle, achieving therapeutic efficacy requires frequent, high-dose applications, which is economically impractical and potentially inefficient.
Hydrogel-based encapsulation systems have therefore emerged as a strategic solution to these delivery challenges, fundamentally addressing the pharmacokinetic limitations of free exosome therapies through enhanced retention and controlled release mechanisms at the wound site.
Hydrogel Mechanisms for Enhanced Exosome Retention and Delivery
Hydrogels, three-dimensional networks of hydrophilic polymers, provide an ideal microenvironment for wound healing by maintaining moisture and mimicking the native extracellular matrix [49] [35]. For exosome delivery, their value lies in two key functional properties: physical retention and controlled release.
The hydrogel's 3D network structure acts as a reservoir, entrapping exosomes within its matrix and preventing their rapid clearance by wound exudate or enzymatic degradation [49]. This physical encapsulation is governed by the hydrogel's pore size, which depends on the polymer type, cross-linking density, and environmental conditions [49]. The sustained and controlled release of exosomes is achieved through a combination of diffusion and hydrogel degradation [49] [40]. As the hydrogel gradually breaks down in the wound environment, it releases exosomes in a temporally controlled manner, ensuring a prolonged therapeutic presence rather than an immediate bolus release.
Furthermore, advanced hydrogel systems can be engineered with environmental responsiveness. For example, hydrogels can be designed to respond to specific wound microenvironment cues such as pH, enzyme activity, or temperature, creating an intelligent release system that delivers exosomes precisely when and where needed [49].
Table 1: Hydrogel Properties Affecting Exosome Retention and Release
| Hydrogel Property | Impact on Exosome Delivery | Key Influencing Factors |
|---|---|---|
| Pore Size | Determines loading efficiency and release rate; smaller pores slow diffusion. | Polymer type, cross-linking agent concentration, cross-linking method [49]. |
| Degradation Rate | Controls the duration of exosome release; must match tissue regeneration speed. | Polymer biodegradability (e.g., chitosan, hyaluronic acid) and cross-link density [40]. |
| Water Content & Swelling | Affects the diffusion coefficient of exosomes through the hydrogel matrix. | Hydrophilicity of the polymer chains and network structure [49]. |
| Dynamic Cross-linking | Enables injectability and self-healing, maintaining integrity under wound stress. | Use of dynamic bonds (e.g., borate ester bonds in PVA/gelatin hydrogels) [36]. |
Quantitative Analysis of Performance Enhancement
Research consistently demonstrates that hydrogel encapsulation significantly improves the pharmacokinetic profile and therapeutic efficacy of exosomes in wound models. The following table summarizes key performance metrics from experimental studies.
Table 2: Quantitative Outcomes of Hydrogel-Encapsulated vs. Free Exosomes in Wound Healing
| Performance Metric | Free Exosomes | Hydrogel-Encapsulated Exosomes | Experimental Context |
|---|---|---|---|
| Retention Time at Wound Site | Rapid clearance; hours to a few days [49] [50]. | Sustained presence over several days to weeks, matching healing timeline [40]. | Various in vivo wound healing models [49] [40]. |
| Angiogenesis | Moderate improvement. | Significantly enhanced capillary density and VEGF expression [49] [24]. | Diabetic rat wound model; MSC-exosome/hydrogel group showed superior results [49]. |
| Wound Closure Rate | Faster than control, but slower than encapsulated forms. | ~95% closure in 14 days (vs. ~80% with free exosomes) [36]. | Full-thickness wound model in male rats using EBO-laden hydrogel [36]. |
| Anti-inflammatory Effect | Moderate reduction of pro-inflammatory factors. | Significant downregulation of TNF-α and IL-1β; promoted M1-to-M2 macrophage polarization [24]. | Chronic wound models with dysregulated inflammation [24]. |
| Targeting & Intracellular Delivery | Compromised delivery efficiency under hypoxia [36]. | Hydrogel alleviates hypoxia, enhancing intracellular cargo delivery of exosomes [36]. | Study using exosome-coated oxygen nanobubbles in hydrogel [36]. |
Experimental Protocols for Evaluating Exosome-Hydrogel Systems
Protocol: Fabrication of an Injectable MSC-Exosome-Laden Hydrogel
This protocol outlines the methodology for creating a chitosan-based hydrogel loaded with MSC-derived exosomes, suitable for in vivo wound dressing applications [49] [51].
Reagents and Materials:
- Mesenchymal Stem Cells (from bone marrow, adipose tissue, or umbilical cord)
- Chitosan powder (high degree of deacetylation)
- Acetic acid solution (1% v/v)
- Glycerol phosphate (optional, for thermal sensitivity)
- Cell culture media and exosome isolation reagents
- Phosphate Buffered Saline (PBS)
- Sterile syringes and 22G needles
Procedure:
- Exosome Isolation: Culture MSCs in serum-free media for 48 hours. Collect the conditioned medium and isolate exosomes via sequential ultracentrifugation (100,000 × g for 70 minutes) or size-exclusion chromatography. Characterize exosomes by Nanoparticle Tracking Analysis (NTA) for size/concentration and Western Blot for markers (CD63, CD81, TSG101) [48] [13].
- Hydrogel Precursor Preparation: Dissolve chitosan powder (1.5-2% w/v) in a 1% acetic acid solution under constant stirring at room temperature until a clear solution is obtained. Adjust the pH to neutral (∼7.0) using a cold sodium hydroxide solution or glycerol phosphate to induce a sol-gel transition [51].
- Exosome Encapsulation: Re-suspend the purified exosome pellet in a small volume of sterile PBS. Gently mix the exosome suspension with the neutralized chitosan hydrogel precursor at a defined ratio (e.g., 10^10 exosomes per 1 mL of hydrogel) by slow pipetting to avoid shear stress.
- Cross-linking and Storage: The resulting mixture can be drawn into a syringe and stored at 4°C until use. The hydrogel forms upon injection and warming to body temperature. The final product should be used within 24 hours for optimal bioactivity.
Protocol: In Vivo Assessment of Retention and Efficacy in a Rodent Wound Model
This protocol describes how to evaluate the performance of the exosome-laden hydrogel in a full-thickness cutaneous wound model [36].
Experimental Groups:
- Control (no treatment)
- Free MSC-derived exosomes (suspended in PBS, applied topically)
- Blank hydrogel (without exosomes)
- MSC-Exosome-laden hydrogel
Procedure:
- Wound Creation: Anesthetize male Sprague-Dawley rats (8-10 weeks old). Create one or two full-thickness excisional wounds (e.g., 8 mm diameter) on the shaved dorsum using a biopsy punch.
- Treatment Application: Apply the prepared treatments (200 µL volume or sufficient to cover the wound) to the respective wounds immediately after creation. Cover the wound with a transparent film dressing.
- Wound Closure Monitoring: Photograph the wounds daily from a fixed distance and height. Use image analysis software (e.g., ImageJ) to quantify the wound area over time. Calculate the percentage of wound closure as:
(Initial Area - Current Area) / Initial Area × 100%. - Tissue Collection and Analysis: Euthanize animals at predetermined time points (e.g., day 7 and 14).
- Histology: Process wound tissue for H&E staining to measure epithelial gap and Masson's Trichrome staining to assess collagen deposition.
- Immunohistochemistry: Stain tissue sections for CD31 to quantify neovascularization and for specific macrophage markers (e.g., CD86 for M1, CD206 for M2) to evaluate immune modulation.
- Exosome Tracking: For retention studies, label exosomes with a lipophilic fluorescent dye (e.g., DiR or PKH67) prior to encapsulation. Use an in vivo imaging system (IVIS) to track the fluorescence signal at the wound site over several days to compare the retention of free vs. encapsulated exosomes.
Visualizing the Workflow and Mechanism
The following diagram illustrates the integrated experimental workflow and the functional mechanism of the exosome-laden hydrogel in the wound environment.
The Scientist's Toolkit: Essential Research Reagents and Materials
The following table catalogues critical materials and reagents required for developing and testing hydrogel-exosome therapies for wound healing.
Table 3: Essential Research Reagents for Hydrogel-Exosome Wound Therapy Research
| Reagent/Material | Function/Application | Examples & Notes |
|---|---|---|
| Mesenchymal Stem Cells (MSCs) | Source of therapeutic exosomes. | Isolate from bone marrow, adipose tissue, or umbilical cord. Characterize by surface markers (CD90+, CD105+, CD73+, CD34-, CD45-) [48] [13]. |
| Hydrogel Polymers | Forms the 3D scaffold for exosome encapsulation. | Natural: Chitosan [51], Hyaluronic Acid [49], Gelatin [36], Alginate. Synthetic: Polyethylene Glycol (PEG), Pluronic F-127 [49]. |
| Exosome Isolation Kits | Purifies exosomes from cell culture media. | Ultracentrifugation is gold standard; commercial kits (e.g., based on precipitation or size-exclusion) offer alternatives [48] [13]. |
| Nanoparticle Tracker | Characterizes exosome size, concentration, and stability. | Instruments like Malvern Nanosight or ZetaView for NTA and DLS analysis [36]. |
| Fluorescent Cell Labels | Tracks exosome retention and cellular uptake in vitro and in vivo. | Lipophilic dyes (e.g., PKH67, DiR); use according to manufacturer's protocol with proper controls [36]. |
| Animal Wound Model | In vivo testing of therapeutic efficacy. | Rodent full-thickness excisional wound model is standard [36]. Ensure approval from institutional animal care and use committee (IACUC). |
The efficacy of mesenchymal stem cell-derived exosomes (MSC-Exos) in wound healing is significantly compromised by their rapid clearance and limited retention at the injury site. Hydrogel-based delivery systems present an ideal solution to this challenge, providing a protective, three-dimensional environment that can be precisely engineered for sustained therapeutic release. The "tunability" of hydrogels—the ability to systematically adjust their mechanical strength, porosity, and degradation kinetics—is fundamental to developing advanced wound therapies [16] [52]. By optimizing these physical properties, researchers can control the diffusion of exosomes from the hydrogel matrix, ensuring a prolonged presence at the wound site that aligns with the complex and protracted process of tissue repair [16] [19]. This Application Note provides a structured framework for designing and characterizing hydrogels tailored for the sustained release of MSC-Exos, consolidating key quantitative data, detailed protocols, and material selection guidelines to accelerate research in this field.
Key Property Interrelationships and Design Goals
The core objective in formulating an MSC-Exos hydrogel is to balance three interdependent physical properties: mechanical strength (to maintain structural integrity against wound bed stresses), porosity (to govern exosome diffusion rates and enable cell infiltration), and degradation rate (to synchronize matrix resorption with tissue regeneration and provide release cues). Adjusting one property invariably impacts the others; for instance, increasing crosslinking density to enhance mechanical strength typically reduces average pore size and may slow down degradation [52]. The design goal is not to maximize any single parameter, but to find an optimal combination that matches the kinetics of wound healing. The following diagram illustrates how these core properties interrelate to ultimately control the release profile of exosomes.
Quantitative Design Parameters for Hydrogel Tuning
Successful formulation requires a quantitative understanding of how processing variables influence final hydrogel properties. The tables below consolidate key data from recent studies to guide the selection of materials and fabrication parameters.
Table 1: Tunable Mechanical and Swelling Properties of Natural Polymer-Based Hydrogels
| Polymer System | Crosslinking / Composite Strategy | Tensile Strength (kPa) | Swelling Ratio (g water/g polymer) | Key Influencing Factors |
|---|---|---|---|---|
| Chitosan | Single-component | ~115 kPa | 800-1500% | Molecular weight, degree of deacetylation [53] |
| Gelatin | Single-component | < 100 kPa | Not Reported | Bloom strength, concentration [53] |
| Chitosan-PVA Composite | Blended synthetic polymer (1:1 to 3:1 mass ratio) | 400-1200 kPa | Not Reported | Mass ratio, crosslinker concentration [53] |
| Recombinant Human Collagen (RHCMA) | Methacrylation & UV crosslinking | 48-137 kPa | 7.3-14.5 | Pre-gel concentration (10-17.5% w/v) [19] |
Table 2: Strategies for Controlling Porosity and Degradation
| Property | Tuning Strategy | Achievable Range | Impact on Release |
|---|---|---|---|
| Porosity & Pore Size | Foam-Templating with surfactants (e.g., PF68-DA/SDS) [54] | Pores < 25 µm to > 50 µm | Smaller, less interconnected pores slow down diffusion. |
| Polymer Concentration (e.g., in RHCMA) [19] | Micropore size decreases with concentration | Higher concentration reduces pore size and swelling, modulating release. | |
| Degradation Time | Succinamide Ester Content (cyclization degradation) [55] | Hours to tens of days | Directly controls release duration via backbone erosion. |
| Blending PEGNBCA/PEGNB (hydrolytic degradation) [56] | Under 2 days to over 3 months | Enables multi-modal release profiles. | |
| Crosslinking Density (e.g., Genipin concentration in CMCS) [57] | 1%, 3%, 5% crosslinker | Higher crosslinking density slows degradation and release. |
Experimental Protocols for Fabrication and Characterization
Protocol: Foam-Templated Fabrication of Microcellular Hydrogel Foams
This protocol describes the creation of porous hydrogels with tunable pore size and interconnectivity using a physical foaming method, adapted from Onyembe & Foudazi [54].
- Key Applications: Creating scaffolds for cell infiltration and rapid exosome diffusion.
- Principle: Aqueous solutions of amphiphilic copolymers (e.g., Pluronic F68-DA) and co-surfactants (e.g., SDS) are foamed with an inert gas (N2). The resulting liquid foam is then polymerized via UV light to lock the porous structure.
Materials:
- Pluronic F68-DA: Polymerizable macromer, forms the primary hydrogel network.
- Sodium Dodecyl Sulfate (SDS): Co-surfactant, tunes micellar assembly and pore interconnectivity.
- Photoinitiator: Omnirad 2959, for UV-initiated crosslinking.
- Nitrogen (N2) Gas: For foaming.
Procedure:
- Solution Preparation: Dissolve Pluronic F68-DA and SDS at desired concentrations in deionized water. Add 0.1% (w/v) Omnirad 2959 photoinitiator and stir until fully dissolved.
- Gas Foaming: Load the solution into a syringe connected to a double-syringe setup. Introduce N2 gas and cycle the solution between the two syringes for a set number of cycles (e.g., 10-20 cycles) at a controlled flow rate to generate a uniform liquid foam.
- UV Polymerization: Transfer the liquid foam to a mold and expose to UV light (e.g., 365 nm wavelength) for a defined time to initiate crosslinking and form a stable porous hydrogel.
- Post-Processing: Wash the polymerized hydrogel foam in deionized water to remove unreacted components and surfactants.
Key Parameters for Tuning:
- SDS Concentration (0 - 50x CMC): Governs pore interconnectivity and final water uptake.
- Gas Fraction and Mixing Energy: Directly controls average pore size and distribution.
- Polymer Concentration: Affects mechanical stability and swelling ratio.
Protocol: Characterizing Hydrolytic Degradation Kinetics
This protocol provides a standardized method to quantify hydrogel degradation, which is crucial for predicting exosome release duration in vivo, based on methods from Dimmitt & Lin [56].
- Objective: To measure the mass loss profile of hydrogels under simulated physiological conditions.
- Data Output: Degradation curve (Mass Remaining % vs. Time), used to calculate degradation half-life.
Procedure:
- Sample Preparation: Prepare hydrogel discs of known dimensions (e.g., 10 mm diameter x 2 mm thickness) and record the initial dry mass (Wdryinitial). For swollen hydrogels, record the initial wet mass (Wwetinitial).
- Incubation: Place each sample in a centrifuge tube containing a pre-warmed phosphate-buffered saline (PBS) solution (e.g., 10-15 mL) at pH 7.4 and 37°C. Use an orbital shaker for gentle agitation.
- Mass Measurement: At predetermined time intervals (e.g., days 1, 3, 7, 14, etc.):
- Remove the sample from the PBS and gently blot with lint-free paper to remove surface water.
- Record the wet mass (Wwett).
- Replace the PBS solution with fresh, pre-warmed PBS to maintain sink conditions and pH.
- Final Dry Mass: Once the hydrogel structure has disintegrated or at the end of the study, recover the remaining polymer (if possible) and dry it completely to determine the final dry mass (Wdryfinal).
- Data Analysis: Calculate the mass remaining at each time point using one of two methods:
- For erosive systems: Mass Remaining (%) = (Wdryfinal / Wdryinitial) * 100%.
- For stable networks that swell: Mass Remaining (%) = (Wwett / Wwetinitial) * 100%.
Protocol: In Vitro Exosome Release and Kinetic Profiling
This protocol details the process of loading MSC-Exos into a hydrogel and quantifying their release profile, as utilized in studies like that of the RHCMA hydrogel [19].
- Objective: To quantify the rate and extent of exosome release from the hydrogel matrix over time.
Materials:
- Fluorescently Labeled Exosomes: e.g., DID-labeled ucMSC-Exos [19].
- Release Medium: PBS or simulated wound fluid.
- Microplate Reader or Spectrofluorometer.
Procedure:
- Exosome Loading: Mix the purified MSC-Exos with the hydrogel pre-gel solution. Allow sufficient time for diffusion and interaction with the polymer network. For photopolymerized hydrogels (e.g., GelMA, RHCMA), subsequently crosslink under UV light.
- Release Study Setup: Immerse the exosome-loaded hydrogel in a known volume of release medium (e.g., 1-5 mL) in a tube. Incubate at 37°C with gentle agitation.
- Sampling: At designated time points, withdraw a small aliquot of the release medium (e.g., 200-500 µL) for analysis. Immediately replace with an equal volume of fresh, pre-warmed medium to maintain sink conditions.
- Quantification: Measure the fluorescence intensity of the sampled medium. Use a standard curve of free fluorescent exosomes to convert intensity to exosome concentration.
- Data Analysis: Calculate the cumulative release percentage over time. Model the release kinetics (e.g., zero-order, first-order, Higuchi, Korsmeyer-Peppas) to determine the dominant release mechanism.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for Hydrogel Formulation and Testing
| Reagent / Material | Function / Role | Example Application / Rationale |
|---|---|---|
| Recombinant Human Collagen (RHC/RHCMA) [19] | Biocompatible, tunable natural polymer base. | Mimics human ECM; ideal for wound applications due to low immunogenicity. |
| Gelatin Methacrylate (GelMA) [57] [58] | Photocrosslinkable protein-based hydrogel. | Combines biocompatibility of gelatin with facile UV-tunable crosslinking. |
| Pluronic F68-DA [54] | Polymerizable surfactant for foam templating. | Enables creation of microcellular hydrogel foams with controlled porosity. |
| Genipin [57] | Natural, low-toxicity chemical crosslinker. | Alternative to glutaraldehyde; crosslinks amine-containing polymers (e.g., chitosan, gelatin). |
| Succinamide Ester Monomers [55] | Provides controllable degradation via cyclization. | Allows precise tuning of hydrogel lifetime from hours to weeks. |
| Poly(ethylene glycol)-norbornene (PEGNB) [56] | Synthetic macromer for step-growth hydrogels. | Forms well-defined, degradable networks via thiol-norbornene click chemistry. |
| MSC-Exos (ucMSC, BMSC, ADSC) [16] [19] | Therapeutic cargo for wound healing. | ucMSC-Exos show superior anti-inflammatory and pro-healing effects in some models [19]. |
| DID Lipophilic Tracer [19] | Fluorescent dye for exosome labeling. | Allows tracking and quantification of exosome release in vitro. |
Integrated Workflow: From Design to In Vivo Assessment
The process of developing an optimized MSC-Exos hydrogel system involves a cyclical process of design, fabrication, characterization, and validation. The following workflow maps out the key experimental stages and decision points.
Concluding Remarks
The strategic tuning of hydrogel properties is a critical determinant for the success of MSC-Exos-based wound therapies. By applying the quantitative design parameters, experimental protocols, and material selection guidelines outlined in this document, researchers can systematically engineer hydrogel platforms that meet the specific demands of different wound healing phases. The interplay between mechanical strength, porosity, and degradation must be empirically determined for each unique formulation, but the foundational data and methods provided here serve as a robust starting point. Future directions will involve the development of even more dynamic "smart" hydrogels that respond to specific wound microenvironment cues (e.g., pH, enzyme activity) for truly autonomous and personalized release profiles.
Mesenchymal stem cell-derived exosomes (MSC-exosomes) hold immense promise as cell-free therapeutics for regenerative medicine, mirroring the therapeutic effects of their parent cells—such as immunomodulation, anti-inflammatory action, and tissue repair—while presenting a safer profile with lower risks of immune rejection and tumorigenicity [59] [60]. However, natural exosomes face significant translational challenges, including limited production yield, rapid clearance from the body, insufficient targeting capability to injury sites, and inherent variability in their therapeutic potency [59] [3].
To overcome these limitations, bioengineering strategies have emerged to enhance the native capabilities of exosomes. These approaches can be broadly categorized into two paradigms: (1) preconditioning of parent MSCs to modulate exosome cargo and enhance biological activity, and (2) direct engineering of exosomes to optimize their therapeutic payload and targeting specificity [59]. When these enhanced exosomes are integrated into hydrogel-based delivery systems, a powerful therapeutic platform is created, enabling sustained and localized release that is particularly advantageous for complex wound healing environments [61] [62]. This document outlines key protocols and application notes for the development and utilization of engineered exosomes within the context of advanced wound therapy.
Preconditioning Strategies for Cargo Enhancement
Preconditioning alters the culture environment of MSCs to induce a phenotypic shift that enhances the therapeutic quality of secreted exosomes. The following table summarizes major preconditioning approaches and their impacts on exosomal cargo.
Table 1: Preconditioning Strategies for MSCs to Enhance Exosome Potency
| Strategy | Typical Protocol | Key Cargo Alterations | Resultant Functional Enhancement |
|---|---|---|---|
| Hypoxia | Culture in 1-3% O₂ for 24-48 hours [59]. | Upregulation of pro-angiogenic miRNAs (e.g., miR-126, miR-210) and proteins (VEGF) [59] [62]. | Enhanced angiogenesis; improved wound healing rates (~30% increase in rodent models) [62]. |
| Inflammatory Cytokine Priming | Treatment with low-dose TNF-α (10-20 ng/mL) or IL-1β (10 ng/mL) for 24 hours [60]. | Significant increase in anti-inflammatory miRNAs (e.g., miR-146a, miR-181a) [59] [60]. | Potent immunomodulation; promotion of M2 macrophage polarization; resolution of inflammation [60]. |
| 3D Culture | Culture as spheroids or in scaffolds/hydrogels for 48-72 hours [59]. | Altered miRNA profile (e.g., increased let-7 family miRNAs); higher yield of exosomes compared to 2D culture [59]. | Improved efficacy in kidney injury and central nervous system disease models [59]. |
| Chemical Preconditioning (LPS) | Stimulation with low-dose LPS (0.1-1 μg/mL) for 24 hours [60]. | Dose-dependent miRNA changes (e.g., upregulation of miR-222-3p, miR-181a-5p) [60]. | Enhanced mitigation of inflammatory damage [60]. |
Application Note: Hypoxic Preconditioning Protocol
Title: Induction of Pro-Angiogenic Exosomes via Hypoxic Preconditioning. Objective: To enhance the angiogenic potential of MSC-exosomes for treating ischemic wounds. Materials:
- Mesenchymal Stem Cells: Human umbilical cord MSCs (ucMSCs) or adipose-derived MSCs (ADSCs) at passage 3-5.
- Hypoxia Chamber: A tri-gas incubator capable of maintaining 1% O₂, 5% CO₂, and balance N₂.
- Culture Media: Serum-free MSC medium.
Methodology:
- Cell Culture: Seed MSCs at 70% confluence in standard culture conditions (37°C, 5% CO₂, 21% O₂).
- Preconditioning: Once cells reach 80-90% confluence, replace medium with fresh serum-free medium and place the culture in the hypoxia chamber set to 1% O₂ for 48 hours [59] [62].
- Exosome Isolation: Collect the conditioned medium. Isolate exosomes via differential ultracentrifugation:
- Centrifuge at 300 × g for 10 min to remove cells.
- Centrifuge supernatant at 2,000 × g for 20 min to remove dead cells.
- Centrifuge supernatant at 10,000 × g for 30 min to remove cell debris.
- Ultracentrifuge supernatant at 100,000 × g for 70 min to pellet exosomes.
- Wash pellet in PBS and repeat ultracentrifugation (100,000 × g, 70 min) [61] [19].
- Validation: Confirm increased potency via tube formation assay using Human Umbilical Vein Endothelial Cells (HUVECs) and qPCR analysis for miR-126 and miR-210.
Direct Engineering Strategies for Functionality
Direct engineering strategies offer precise control over exosome composition and function, enabling the creation of "designer" vesicles for specific therapeutic applications.
Table 2: Direct Engineering Strategies for MSC-Exosomes
| Engineering Approach | Methodology | Key Outcomes | Considerations |
|---|---|---|---|
| Cargo Loading | Electroporation: Mix exosomes with therapeutic miRNA/siRNA and apply electrical pulses (~350 mV, 150 ms).Transfection: Transfect parent MSCs with plasmid DNA/mimics to load exosomes endogenously [59]. | Efficient delivery of osteogenic miRNAs (e.g., miR-196a) or anti-fibrotic miRNAs (e.g., miR-192-5p) [3] [63]. | Electroporation can cause exosome aggregation; transfection ensures natural loading but efficiency varies [59]. |
| Surface Functionalization | Genetic Engineering: Transduce MSCs to express exosome surface proteins (e.g., Lamp2b) fused with targeting peptides (e.g., RGD, iRGD).Chemical Conjugation: Use click chemistry to conjugate targeting ligands (e.g., c(RGDyK) peptide) to amine groups on exosome surface proteins [59] [63]. | Enhanced retention at wound site; improved uptake by specific cell types (e.g., fibroblasts, keratinocytes) [63]. | Genetic methods are more consistent; chemical methods require optimization to preserve membrane integrity. |
Application Note: Surface Functionalization for Targeted Delivery
Title: Genetic Engineering of Exosomes for Enhanced Wound Site Targeting. Objective: To display a targeting peptide on the exosome surface to improve its retention and uptake in wound tissue. Materials:
- Plasmid Vector: Encoding a fusion protein of exosomal membrane protein (e.g., Lamp2b) and a targeting peptide (e.g., RGD for integrin targeting).
- Transduction Reagents: Lentiviral packaging system or polyethylenimine (PEI).
- Parent Cells: HEK293T cells for lentivirus production; MSCs for exosome production.
Methodology:
- Virus Production: Package the targeting plasmid into lentivirus using HEK293T cells.
- Cell Transduction: Transduce MSCs with the lentivirus and select with puromycin to create a stable cell line.
- Exosome Production: Culture the engineered MSCs and isolate exosomes via ultracentrifugation as described in Section 2.1.
- Validation: Confirm peptide display via flow cytometry (using an antibody against the peptide tag) and test targeting efficiency in a co-culture assay with fibroblasts and keratinocytes.
Hydrogel Encapsulation for Sustained Wound Release
Hydrogels provide a protective, three-dimensional scaffold that enriches exosomes at the wound site and controls their release kinetics, which is critical for the prolonged healing process.
Application Note: Fabrication of an Exosome-Loaded Recombinant Human Collagen Hydrogel
Title: Encapsulation of Engineered Exosomes in Methacrylated Recombinant Human Collagen Hydrogel (RHCMA) for Wound Dressing. Objective: To create a biocompatible, sustained-release exosome delivery system for topical wound application. Materials:
- Recombinant Human Collagen (RHC)
- Methacrylic Anhydride (MA)
- Photoinitiator: Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP)
- UV Light Source (365 nm, 5-10 mW/cm²)
- Engineered Exosomes (e.g., hypoxia-preconditioned ucMSC-exosomes)
Methodology:
- Synthesis of RHCMA: Modify RHC by reacting with methacrylic anhydride at an optimal concentration to introduce photo-crosslinkable methacrylate groups onto the collagen backbone. Purify the resulting RHCMA product [61] [19].
- Preparation of Pre-gel Solution: Dissolve RHCMA in PBS at a 10% (w/v) concentration. Add the LAP photoinitiator at 0.05% (w/v). This concentration offers an ideal balance of mechanical strength and release kinetics [61] [19].
- Loading Exosomes: Gently mix the engineered exosomes into the RHCMA pre-gel solution to achieve a homogeneous suspension.
- Crosslinking: Pipette the exosome-loaded pre-gel solution onto the wound bed and expose to UV light (365 nm) for 2-5 minutes to form a stable, crosslinked hydrogel in situ [61] [19].
Release Kinetics: The 10% RHCMA hydrogel exhibits a classic sustained release profile, with an initial release of ~56% of exosomes within the first 12 hours, followed by a gradual release stabilizing after 48 hours (cumulative release ~92%) [61]. This pattern ensures both an immediate biological effect and a prolonged therapeutic presence.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for Exosome Engineering and Hydrogel Encapsulation
| Reagent / Material | Function | Example & Notes |
|---|---|---|
| Recombinant Human Collagen (RHC) | Base material for biocompatible hydrogel; avoids animal-derived collagen risks. | Source: Recombinant expression systems. Superior biocompatibility and promotes ECM remodeling [61] [19]. |
| Methacrylic Anhydride (MA) | Introduces methacrylate groups for photo-crosslinking of hydrogels. | Used to synthesize RHCMA; concentration must be optimized for mechanical properties [61] [19]. |
| LAP Photoinitiator | Initiates polymerization under non-invasive UV light for in situ gelation. | Lithium phenyl-2,4,6-trimethylbenzoylphosphinate. Preferable for its cytocompatibility [61]. |
| Lentiviral Vectors | For stable genetic modification of parent MSCs to engineer exosome cargo or surface. | Allows for consistent production of engineered exosomes from a stable cell line [59] [63]. |
| Ultracentrifuge | Gold-standard equipment for isolating high-purity exosomes from conditioned media. | Critical for obtaining research-grade exosomes free of contaminating proteins [61] [3]. |
| CD9, CD63, CD81 Antibodies | Canonical exosome markers for characterization and quantification (per MISEV2023 guidelines). | Used in Western Blot, flow cytometry, or ELISA to confirm exosome identity [63]. |
Concluding Remarks
The convergence of exosome engineering and advanced biomaterial design represents a paradigm shift in regenerative therapeutics. By systematically applying preconditioning and direct engineering strategies, researchers can tailor the inherent properties of exosomes to address specific pathological aspects of wound healing. Subsequent encapsulation within a tunable hydrogel matrix ensures that these enhanced capabilities are delivered to the wound site in a sustained and protected manner. The protocols outlined herein provide a foundational framework for developing and testing such integrated therapeutic systems, paving the way for more effective and predictable treatments for chronic and acute wounds.
The convergence of mesenchymal stem cell (MSC)-derived exosomes with hydrogel delivery systems represents a paradigm shift in regenerative medicine, particularly for chronic wound treatment. These cell-free therapies harness the pro-regenerative, immunomodulatory, and angiogenic properties of MSC-exosomes while utilizing hydrogels to overcome critical delivery challenges such as rapid clearance and limited retention at the wound site [16]. However, the transition from promising preclinical results to clinically viable and widely available therapies faces substantial scalability and production hurdles. These challenges span the entire development pipeline, from exosome biomanufacturing and hydrogel design to functional integration and quality control. This document provides a detailed analysis of these translational bottlenecks and offers standardized protocols to support consistent, scalable production of MSC-exosome hydrogel therapies, equipping researchers and development professionals with practical tools for navigating this complex landscape.
Critical Analysis of Scalability Challenges
The scalable production of MSC-exosome hydrogel therapies is constrained by several interdependent factors that impact both the biological active ingredient (exosomes) and the delivery matrix (hydrogel).
Exosome Production and Standardization Bottlenecks
The upstream production of MSC-exosomes presents the most significant scalability challenges, primarily due to the inherent biological variability of donor-derived MSCs and the technical limitations of conventional culture and isolation methods.
- Source-Dependent Variability: Different MSC sources yield exosomes with distinct therapeutic profiles, directly impacting the consistency of the final product. Comparative studies reveal that exosomes derived from human umbilical cord MSCs (ucMSC-exos) demonstrate superior acceleration of wound healing compared to those from adipose-derived (ADSC-exos) or bone marrow-derived MSCs (BMSC-exos), attributed to enhanced inflammatory resolution and collagen formation [19]. This variability necessitates rigorous source selection and qualification.
- Isolation Method Limitations: The most common isolation method, differential ultracentrifugation, is difficult to scale, suffers from low yield, and can compromise exosome integrity due to high shear forces [64]. While alternative methods like ultrafiltration, density gradient centrifugation, and anion exchange chromatography offer improved scalability and purity, they introduce new challenges related to standardization, cost, and validation for Good Manufacturing Practice (GMP) compliance [64].
- Quantification and Characterization: A critical barrier to standardization is the lack of unified metrics for exosome quantification. Common methods like nanoparticle tracking analysis (NTA) provide particle concentration but not a functional dose. Establishing a correlation between particle number, protein content (e.g., via BCA assay), and in vitro bioactivity is essential for defining a reproducible therapeutic dose [19] [36].
Table 1: Quantitative Comparison of MSC-Exosome Sources for Wound Healing
| Exosome Source | Average Diameter (nm) | Key Therapeutic Strengths | Notable Cargo/Markers | Relative Wound Healing Efficacy (Preclinical) |
|---|---|---|---|---|
| Umbilical Cord MSC (ucMSC-exos) | 103.1 ± 3.8 [19] | Anti-inflammatory, Macrophage regulation, Collagen remodeling [19] | TSG-6, IL-10 [16] | Superior (Best performance in rat model) [19] |
| Adipose-Derived MSC (ADSC-exos) | 103.8 ± 3.3 [19] | Angiogenesis, Fibroblast migration [19] [36] | VEGF, TGF-β, miR-31 [16] | High [19] [36] |
| Bone Marrow MSC (BMSC-exos) | 102.6 ± 3.4 [19] | Cell proliferation, Anti-apoptosis [19] | miR-23a-3p, miR-219-5p [16] | High [19] |
Hydrogel Manufacturing and Exosome Integration Hurdles
The encapsulation of exosomes into hydrogels introduces another layer of complexity for scale-up, focusing on preserving exosome bioactivity and achieving controlled release kinetics.
- Bioactivity Preservation: The hydrogel crosslinking process, whether chemical (e.g., photo-polymerization of GelMA) or physical (e.g., borate bonds in PVA/gelatin), must maintain a mild environment to prevent denaturation of sensitive exosomal proteins and nucleic acids [47] [36]. The choice of polymer (natural vs. synthetic) directly impacts this biocompatibility.
- Release Kinetics Control: A primary therapeutic advantage of hydrogels is sustained exosome release. Research shows that a 10% recombinant human collagen hydrogel (RHC) can release over 90% of its encapsulated exosomes within 48 hours in vitro [19]. While rapid, this must be tuned to match the prolonged healing process of chronic wounds. Hydrogel properties such as polymer concentration, pore architecture, and degradation rate are critical levers for controlling release profiles [65] [19].
- Sterilization and Shelf-Life: Terminal sterilization of the final product (e.g., gamma irradiation) can damage both the hydrogel structure and exosome cargo. Aseptic processing is often required but increases production cost and complexity. Furthermore, the long-term stability of the combined product (exosome-loaded hydrogel) requires extensive validation of storage conditions to ensure functional activity is retained [65].
Table 2: Scalability Assessment of Common Hydrogel Polymers for Exosome Delivery
| Polymer Type | Example Formulation | Key Scalability Considerations | Impact on Exosome Activity |
|---|---|---|---|
| Natural Polymers | GelMA-Dopamine [47], Recombinant Human Collagen (RHC) [19] | Batch-to-batch variability of animal-derived materials (e.g., gelatin). Cost of recombinant proteins. Advantage: Innate bioactivity. | High biocompatibility; supports bioactivity and cell infiltration [47] [19]. |
| Synthetic Polymers | Polyvinyl Alcohol (PVA) [36] | High reproducibility. Low bioactivity may require functionalization. Scalable and cost-effective. | Requires incorporation of bioactive motifs (e.g., gelatin [36]) to enhance cell-exosome interactions. |
| Hybrid Systems | PVA/Gelatin/Borax [36] | Balances reproducibility (PVA) with bioactivity (gelatin). Process development for consistent mixing. | Favorable; provides structural stability and a bioactive microenvironment [65] [36]. |
Experimental Protocols for Scalability and Functional Assessment
To address these hurdles, standardized and scalable protocols are essential. The following sections provide detailed methodologies for key processes.
Protocol: Scalable Isolation of MSC-Exosomes via Tangential Flow Filtration (TFF)
Application Note: This protocol is designed to replace ultracentrifugation for larger-volume MSC-conditioned media processing, offering higher yield, better reproducibility, and improved preservation of exosome integrity, which is critical for GMP translation [64].
Materials:
- Source: Conditioned media from human ucMSCs (passage 4-6) grown in serum-free media.
- TFF System: Benchtop TFF system with a 100-300 kDa molecular weight cut-off (MWCO) hollow fiber filter or cassette.
- Buffers: Dulbecco's Phosphate-Buffered Saline (DPBS), pH 7.4.
- Consumables: 0.22 µm PES sterile filters, storage vials.
Procedure:
- Harvest and Clarification: Collect conditioned media from ucMSCs. Perform an initial centrifugation at 2,000 × g for 30 minutes at 4°C to remove cells and large debris. Filter the supernatant through a 0.22 µm PES filter.
- TFF System Setup and Priming: Aseptically install the TFF filter according to the manufacturer's instructions. Prime the system with DPBS to remove preservatives and check for leaks.
- Concentration and Diafiltration: Load the clarified media into the TFF reservoir. Concentrate the retentate to approximately 1/20 of the original volume. Initiate diafiltration by continuously adding DPBS to the retentate at the same rate as the permeate flow. Perform a volume exchange of 5-10 diavolumes to effectively remove contaminating soluble proteins.
- Final Recovery: Once diafiltration is complete, concentrate the retentate to a final volume of 10-20 mL. Recover the concentrated exosome solution and aliquot into sterile vials.
- Characterization: Analyze the final product for particle concentration (NTA), size distribution (DLS), protein content (BCA assay), and purity (Western Blot for CD9, CD63, CD81) [19] [64].
Protocol: Fabrication of a Scalable GelMA-Dopamine Hydrogel Loaded with Exosomes
Application Note: This protocol describes the synthesis of a photocrosslinkable, adhesive hydrogel suitable for encapsulating exosomes. The formulation is designed for ease of mixing and injection, facilitating clinical application [47].
Materials:
- Polymers: Methacrylated Gelatin (GelMA, 10% w/v), Dopamine hydrochloride.
- Photo-initiator: Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP, 0.1% w/v).
- Exosomes: ucMSC-exosomes isolated via TFF, resuspended in PBS.
- Equipment: UV light source (365 nm, 5-10 mW/cm²), vortex mixer.
Procedure:
- Hydrogel Precursor Preparation: Dissolve GelMA powder in DPBS at 40°C to prepare a 10% (w/v) stock solution. Add dopamine hydrochloride to the GelMA solution at a final concentration of 0.5-1 mg/mL and vortex until fully dissolved.
- Exosome Incorporation: Thaw the exosome suspension on ice. Add the calculated volume of exosome suspension to the GelMA-Dopamine precursor solution. Gently mix by pipetting to avoid shear stress. Add the LAP photo-initiator and mix thoroughly.
- Crosslinking and Curing: Transfer the exosome-loaded precursor mixture to the desired mold or directly onto the wound bed. Expose the mixture to UV light (365 nm) for 30-60 seconds to initiate crosslinking and form a stable hydrogel.
- Quality Control: Assess the mechanical properties of the cured hydrogel via compression testing. Confirm the in vitro sustained release profile by incubating the hydrogel in PBS at 37°C and measuring exosome concentration in the supernatant over time using NTA or a fluorescence-based assay [47] [19].
The Scientist's Toolkit: Essential Research Reagents and Materials
A summary of key materials, their functions, and scalability considerations is provided below.
Table 3: Research Reagent Solutions for Exosome-Hydrogel Therapy Development
| Reagent/Material | Function in Workflow | Scalability & Sourcing Considerations |
|---|---|---|
| Umbilical Cord MSCs | Cellular source for exosome production. Preferred for high anti-inflammatory and pro-healing activity [19]. | Source from reputable cell banks. Master cell banks and standardized culture protocols are essential for batch consistency. |
| Tangential Flow Filtration (TFF) System | Scalable isolation and concentration of exosomes from large volumes of conditioned media [64]. | Prefer single-use flow paths to minimize cross-contamination and validation efforts. |
| Methacrylated Gelatin (GelMA) | Photocrosslinkable hydrogel polymer providing a bioactive 3D matrix for exosome encapsulation and cell growth [47] [19]. | Opt for GMP-grade or recombinant GelMA to avoid animal-derived component variability. |
| Lithium Phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) | A biocompatible photo-initiator for UV-mediated hydrogel crosslinking [47]. | Sourced from chemical suppliers; ensure high purity for consistent gelation kinetics and cytocompatibility. |
| Recombinant Human Collagen (RHC) | A highly pure and consistent alternative to animal-derived collagen for hydrogel fabrication [19]. | More expensive than animal collagen but offers superior lot-to-lot consistency and reduced immunogenicity risk. |
| Nanoparticle Tracking Analyzer (NTA) | Instrument for determining exosome particle size distribution and concentration [19] [36]. | Critical for Quality Control (QC); requires standardized operating procedures to ensure inter-laboratory reproducibility. |
Visualization of Workflows and Signaling Pathways
From Bioprocessing to Clinical Application: A Scalable Workflow
The following diagram outlines the integrated production pipeline, highlighting critical scalability checkpoints and quality control stages from cell culture to final product application.
Diagram 1: Integrated scalable workflow for producing exosome-laden hydrogels, highlighting critical checkpoints for process scaling and quality control.
Mechanistic Pathways of MSC-Exosomes in Diabetic Wound Healing
The therapeutic efficacy of released exosomes in the complex diabetic wound microenvironment is mediated through the modulation of key cellular processes and signaling pathways.
Diagram 2: Key mechanistic pathways modulated by MSC-exosomes in the diabetic wound microenvironment, leading to functional healing outcomes.
Proof of Concept: Preclinical Validation and Comparative Analysis of Therapeutic Outcomes
The management of diabetic and chronic wounds remains a significant clinical challenge, characterized by impaired healing processes such as prolonged inflammation, inadequate angiogenesis, and cellular dysfunction [66]. Traditional treatments often fail to address the complex pathophysiology of these wounds, necessitating innovative therapeutic strategies. Among these, mesenchymal stem cell (MSC)-derived exosomes have emerged as a promising cell-free therapy, capable of modulating the wound microenvironment through their rich cargo of bioactive molecules [67] [50]. However, the clinical translation of exosome-based therapies is hindered by their rapid clearance and limited retention at the wound site [16].
To overcome these limitations, hydrogel-based delivery systems have been developed to provide a sustained release of exosomes, thereby enhancing their therapeutic efficacy [62] [36]. This application note synthesizes current preclinical evidence on the in vivo performance of hydrogel-exosome systems, providing a comprehensive analysis of their efficacy, detailed protocols for implementation, and an examination of the underlying molecular mechanisms in diabetic and chronic wound models.
In Vivo Efficacy Data in Preclinical Models
Recent studies have demonstrated the superior performance of hydrogel-exosome combinations across various animal models of impaired wound healing. The encapsulated exosomes exhibit prolonged retention and stability, leading to enhanced therapeutic outcomes through multiple mechanisms.
Table 1: Summary of Hydrogel-Exosome System Efficacy in Preclinical Wound Models
| Exosome Source | Hydrogel System | Animal Model | Key Efficacy Findings | Proposed Mechanisms | Citation |
|---|---|---|---|---|---|
| ADSC-derived exosomes | Polyvinyl alcohol/gelatin-borax hybrid hydrogel | Male rat full-thickness wound model | Accelerated wound closure, ameliorated hypoxia, reduced inflammation | Enhanced exosome delivery, oxygen nanobubble-mediated hypoxia alleviation, antioxidant activity | [36] |
| Hypoxia-pretreated ADSC-derived exosomes | Not specified (embedded hydrogel) | Rodent diabetic wound model | ≈30% increased wound healing rate, enhanced angiogenesis | Sustained VEGF delivery, antioxidant, immunomodulatory, and pro-angiogenic activities | [62] |
| ADSC-derived exosomes | Not specified (loaded hydrogel) | Mouse wound model | Accelerated healing, increased M2 macrophage polarization, enhanced collagen deposition and angiogenesis | Macrophage polarization via IL-33 release, activation of Wnt/β-catenin signaling in keratinocytes | [68] |
| BMSC-derived exosomes | Chitosan hydrogel | Bone repair model | Excellent osteogenic properties, promoted bone regeneration | Controlled exosome release, enhanced osteogenic differentiation | [16] |
The data from these studies consistently demonstrate that hydrogel-exosome systems significantly improve healing parameters compared to free exosomes or control treatments. A particularly innovative approach involved a hybrid hydrogel incorporating exosome-coated oxygen nanobubbles (EBO), which addressed both the hypoxic wound environment and the need for efficient exosome delivery [36]. This multifunctional system not only facilitated exosome internalization by cells but also provided oxygen to hypoxic tissues, resulting in accelerated wound closure and reduced inflammation in a rat full-thickness wound model.
Similarly, ADSC-exosomes embedded in hydrogels have shown remarkable efficacy in modulating immune responses. These systems promoted the polarization of macrophages toward the anti-inflammatory M2 phenotype, which in turn increased the release of interleukin-33 (IL-33) [68]. This cytokine enhanced keratinocyte proliferation, collagen deposition, and epithelialization through the Wnt/β-catenin signaling pathway, creating a regenerative microenvironment conducive to healing.
Experimental Protocols
Protocol: Development and Evaluation of an Exosome-Laden Hydrogel System for Diabetic Wounds
This protocol outlines the methodology for creating and testing a self-healing polyvinyl alcohol (PVA)/gelatin (GA) hybrid hydrogel loaded with ADSC-derived exosomes, based on the work published in Nature Communications [36].
Materials:
- Human adipose-derived stem cells (ADSCs)
- Polyvinyl alcohol (PVA)
- Gelatin (GA)
- Borax
- Dio fluorescent dye (for exosome labeling)
- Transmission Electron Microscope (TEM)
- Nanoparticle Tracking Analysis (NTA) system
- Dynamic Light Scattering (DLS) instrument
- Streptozotocin (for inducing diabetes)
- Male Sprague-Dawley rats (for in vivo model)
Procedure:
Step 1: Isolation and Characterization of ADSC-derived Exosomes
- Culture ADSCs in standard medium and passage until sufficient cells are obtained.
- Collect cell culture supernatant and isolate exosomes using sequential ultracentrifugation.
- Centrifuge at 300 × g for 10 min to remove cells.
- Centrifuge at 2,000 × g for 20 min to remove dead cells.
- Centrifuge at 10,000 × g for 30 min to remove cell debris.
- Ultracentrifuge at 100,000 × g for 70 min to pellet exosomes.
- Resuspend exosome pellet in phosphate-buffered saline (PBS).
- Characterize exosomes using:
- NTA: Determine particle size distribution and concentration (approximately 125 nm diameter expected).
- TEM: Confirm cup-shaped morphology.
- Western Blot: Analyze surface markers (CD9, CD63, CD81).
Step 2: Synthesis of Exosome-Laden Hydrogel
- Prepare PVA/GA hydrogel base: Dissolve PVA and GA in deionized water at 90°C with constant stirring. Allow solution to cool to room temperature.
- Crosslinking: Add borax solution dropwise to the PVA/GA mixture under continuous stirring to form dynamic borate ester bonds, enabling self-healing properties.
- Incorporate exosomes: Gently mix the isolated exosomes into the hydrogel matrix at a concentration of 7.22 × 10^8 particles/mL hydrogel precursor.
- Characterize hydrogel properties: Assess rheological properties, swelling behavior, and degradation profile.
Step 3: In Vivo Evaluation in Diabetic Rat Wound Model
- Induce diabetes: Administer streptozotocin (55 mg/kg, intraperitoneally) to male rats. Confirm hyperglycemia (blood glucose >300 mg/dL) after 72 hours.
- Create wounds: Anesthetize diabetic rats and create full-thickness excisional wounds (diameter: 8 mm) on the dorsal skin.
- Apply treatments: Randomly assign animals to four groups (n=6 per group):
- Group 1: No treatment (control)
- Group 2: Hydrogel only
- Group 3: Free ADSC-derived exosomes
- Group 4: ADSC-exosome laden hydrogel
- Monitor wound closure: Capture digital images of wounds on days 0, 3, 7, 10, and 14. Calculate wound area using image analysis software.
- Assess histological and molecular changes:
- Collect wound tissue samples on day 7 and 14 for histological analysis (H&E staining, Masson's trichrome staining).
- Perform immunohistochemistry for CD31 (angiogenesis), CD86 (M1 macrophages), and CD206 (M2 macrophages).
- Analyze gene expression of inflammatory cytokines (IL-6, TNF-α) using qPCR.
Protocol: Assessing Macrophage Polarization Mediated by Hydrogel-Exosome Systems
This protocol details methods for evaluating the immunomodulatory effects of ADSC-exosome hydrogels on macrophage polarization, a key mechanism in promoting wound healing [68].
Materials:
- RAW264.7 cell line (murine macrophages)
- Bone marrow-derived macrophages (BMDMs) from C57BL/6 mice
- IL-4 and IL-13 (for M2 polarization)
- LPS and IFN-γ (for M1 polarization)
- IL-33 recombinant protein
- Antibodies for F4/80, CD86, CD206, and IL-33
Procedure:
Step 1: In Vitro Macrophage Polarization Assay
- Culture RAW264.7 cells or isolate BMDMs from mouse femur and tibia.
- Treat macrophages with:
- LPS (50 ng/mL) + IFN-γ (20 ng/mL) for 24 h to induce M1 phenotype.
- IL-4 (20 ng/mL) + IL-13 (20 ng/mL) for 48 h to induce M2 phenotype.
- ADSC-exosomes (1 × 10^10 particles/well) with or without hydrogel.
- Analyze polarization status using:
- Immunofluorescence: Stain for M1 marker (CD86) and M2 marker (CD206).
- qPCR: Measure expression of M1 genes (iNOS, IL-6) and M2 genes (ARG1, IL-10).
Step 2: In Vivo Validation of Macrophage Polarization
- Generate wounds in diabetic mice and treat with ADSC-exosome hydrogel as in Protocol 3.1.
- Harvest wound tissue at days 7 and 14 post-treatment.
- Analyze macrophage populations by flow cytometry using antibodies against F4/80 (pan-macrophage), CD86 (M1), and CD206 (M2).
- Perform RNA sequencing on isolated wound macrophages to identify differentially expressed genes, particularly IL-33.
Step 3: Functional Validation of IL-33
- Utilize IL-33 knockout (
Il33−/−) mice to confirm the role of IL-33 in ADSC-exosome-mediated healing. - Perform co-culture experiments with macrophages and keratinocytes (HaCat cell line) to assess the effect of IL-33 on keratinocyte proliferation and migration via the Wnt/β-catenin pathway.
Signaling Pathways and Mechanisms of Action
Hydrogel-exosome systems promote healing through multiple coordinated mechanisms. The following diagrams illustrate the key signaling pathways and functional relationships involved.
IL-33/Wnt/β-catenin Signaling in Keratinocyte Activation
Multi-mechanistic Actions of Hydrogel-Exosome Systems
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Research Reagents for Hydrogel-Exosome Wound Healing Studies
| Reagent/Category | Specific Examples | Function/Application | Representative Findings |
|---|---|---|---|
| Exosome Sources | Adipose-Derived Stem Cells (ADSCs), Bone Marrow MSCs (BMSCs), Umbilical Cord MSCs | Provide therapeutic exosomes with pro-regenerative cargo (proteins, miRNAs, lipids) | ADSC-Exos promoted collagen deposition and angiogenesis; BMSC-Exos facilitated bone repair [16] [68]. |
| Hydrogel Polymers | Polyvinyl Alcohol (PVA), Gelatin, Chitosan, Hyaluronic Acid | Form 3D scaffold for sustained exosome release, provide structural support | PVA/gelatin-borax hydrogel showed self-healing, antioxidative, and hemostatic properties [36]. |
| Characterization Tools | Nanoparticle Tracking Analysis (NTA), Transmission Electron Microscopy (TEM), Western Blot | Isolate, quantify, and characterize exosomes (size, concentration, morphology, markers) | Confirmed exosome size of ~125 nm and presence of markers CD9, CD63, CD81 [36]. |
| Animal Models | Streptozotocin-induced diabetic rodents, Genetically diabetic (db/db) mice, Full-thickness excisional wound model | Recapitulate key aspects of human chronic wounds (hyperglycemia, impaired healing) | Hydrogel-exosome systems demonstrated ≈30% increased healing rate in diabetic rodents [62]. |
| Analysis Kits & Reagents | CD31, CD86, CD206 antibodies, IL-33 ELISA/recombinant protein, Masson's Trichrome stain | Assess angiogenesis, macrophage polarization, cytokine levels, and collagen deposition | Confirmed increased M2 macrophages (CD206+) and enhanced collagen deposition in treated groups [68]. |
Hydrogel-exosome systems represent a paradigm shift in the therapeutic approach to diabetic and chronic wounds. The in vivo data comprehensively demonstrate that these systems significantly enhance wound healing through multiple coordinated mechanisms: modulating immune responses via macrophage polarization, promoting angiogenesis, mitigating oxidative stress, and facilitating extracellular matrix remodeling. The sustained release profile provided by hydrogels addresses the critical limitation of rapid exosome clearance, thereby maximizing their regenerative potential at the wound site.
The protocols and mechanistic insights provided in this application note offer researchers a foundation for developing and evaluating advanced hydrogel-exosome formulations. As this field progresses, future research should focus on optimizing exosome dosing, refining hydrogel degradation kinetics to match healing timelines, and conducting large-scale preclinical safety studies to facilitate clinical translation. The integration of these sophisticated delivery systems holds considerable promise for addressing the unmet clinical needs in chronic wound management.
Exosomes derived from mesenchymal stem cells (MSCs) have emerged as a promising cell-free therapeutic strategy in regenerative medicine, offering significant advantages over whole-cell therapies, including enhanced stability, reduced immunogenicity, and the ability to cross biological barriers [69]. These nano-sized extracellular vesicles (30-150 nm) facilitate intercellular communication by delivering a diverse array of bioactive molecules, including proteins, lipids, mRNAs, and microRNAs, to recipient cells [70]. The therapeutic potential of MSC-derived exosomes varies considerably depending on their tissue of origin, with umbilical cord (UC), bone marrow (BM), and adipose tissue (AD) representing the most prominent sources. When framed within the context of hydrogel encapsulation for sustained wound release, understanding these source-dependent differences becomes paramount for optimizing therapeutic efficacy. This comparative analysis examines the biological characteristics, functional mechanisms, and therapeutic applications of exosomes derived from these three sources to guide researchers in selecting the most appropriate exosome type for specific regenerative applications, particularly in advanced hydrogel-based delivery systems.
Biological Characteristics and Composition
Exosomes from different MSC sources exhibit distinct biological profiles that influence their therapeutic potential. These differences stem from the unique microenvironment of their parent cells and result in variations in cargo composition, surface markers, and physical properties.
Table 1: Comparative Characterization of MSC-Derived Exosomes
| Characteristic | Umbilical Cord (UC) | Bone Marrow (BM) | Adipose Tissue (AD) |
|---|---|---|---|
| Size Range | 30-150 nm [70] | 30-150 nm [71] | 30-200 nm [72] |
| Common Markers | CD63, CD81, ALIX [71] | CD63, CD9, CD81 [73] | Tetraspanins (CD63, CD81) [74] |
| Particle Concentration | 1.2 × 10⁸ particles/mL [71] | 6.9 × 10⁷ particles/mL [71] | 8.0 × 10⁷ particles/mL [71] |
| Key miRNA Cargo | miR-29b, miR-133a-3p, miR-24-3p [69] | miR-21, miR-34a [73] | miR-21, miR-146a [74] |
| Protein Content | >400 proteins, HSP70, HSP90, antioxidant enzymes [69] | Proteins modulating NF-κB pathway [71] | Growth factors, cytokines [72] |
| Advantages | High proliferation capacity, strong immunomodulation [71] | Extensive research history, strong chondroprotective effects [71] | Abundant tissue source, easy isolation [72] |
The compositional differences directly influence functional capacities. UC-MSC exosomes contain numerous cytokines, including granulocyte-macrophage colony-stimulating factor, IL-6, IL-8, and IL-10, with particularly high concentrations of IL-6 and IL-8 [69]. BM-MSC exosomes are enriched with molecules that modulate the NF-κB and MAPK signaling pathways [71], while AD-MSC exosomes carry proteins and miRNAs that activate Wnt/β-catenin and PI3K/Akt pathways to promote tissue regeneration [72].
Figure 1: Composition and Functional Differences of MSC-Derived Exosomes. Exosomes from different tissue sources carry distinct molecular cargo that determines their therapeutic specialization.
Functional Efficacy in Disease Models
The therapeutic efficacy of MSC-derived exosomes has been extensively evaluated across various disease models, with source-dependent performance variations observed in anti-inflammatory response, tissue regeneration, and immunomodulation.
Anti-inflammatory Properties
Comparative studies demonstrate significant differences in the anti-inflammatory capabilities of exosomes from different sources. In vitro analyses reveal that UC-MSC and BM-MSC exosomes exhibit superior efficacy in attenuating inflammation compared to AD-MSC exosomes [71]. Treatment with UC-MSC and BM-MSC exosomes significantly reduces phosphorylated p65 (pp65) levels in the NF-κB pathway and decreases phosphorylation of p38 (pp38), JNK (pJNK), and ERK (pERK) in the MAPK pathway following IL-1β stimulation [71]. These molecular changes correspond with reduced expression of pro-inflammatory mediators and enhanced expression of chondroprotective genes.
In acute lung injury models, UC-MSC exosomes administered via various routes (intravenous, intranasal, or nebulization) significantly attenuate pulmonary inflammation, reducing alveolar inflammatory cell infiltration, hemorrhage, and edema [75]. ELISA analyses confirm that UC-MSC exosomes markedly decrease pro-inflammatory cytokines TNF-α, IL-6, and IL-1β while increasing anti-inflammatory IL-10 levels in bronchoalveolar lavage fluid [75].
Tissue Regenerative Capacity
The tissue regenerative potential of MSC-derived exosomes varies by source, with each exhibiting particular strengths in different regenerative contexts:
UC-MSC Exosomes: Demonstrate exceptional wound healing capabilities, significantly accelerating wound closure by reducing inflammation, stimulating angiogenesis, and promoting extracellular matrix formation [70]. In vitro analyses indicate that UC-MSC exosomes significantly promote the proliferation and migration of human skin fibroblasts and enhance tube formation in human umbilical vein endothelial cells [70].
BM-MSC Exosomes: Exhibit strong chondroprotective effects and promote cartilage protection. In osteoarthritis models, BM-MSC exosomes significantly suppress proinflammatory markers while enhancing the expression of chondroprotective genes and inhibiting chondrocyte apoptosis [71].
AD-MSC Exosomes: Promote tissue regeneration through activation of multiple signaling pathways including Wnt/βcatenin and PI3K/Akt, facilitating immunomodulation, angiogenesis, cell migration, proliferation, and tissue remodeling [72]. These exosomes have shown efficacy in wound healing, cardiovascular diseases, neurodegenerative conditions, and skeletal disorders [72].
Table 2: Functional Efficacy Comparison in Disease Models
| Disease Model | UC-MSC Exosomes | BM-MSC Exosomes | AD-MSC Exosomes |
|---|---|---|---|
| Wound Healing | Significant acceleration of wound closure; Enhanced angiogenesis and collagen deposition [70] | Not specifically reported | Promotes proliferation and migration; Modulates inflammation [74] |
| Osteoarthritis | Superior efficacy in attenuating inflammation and promoting cartilage protection [71] | Suppresses proinflammatory markers; Enhances chondroprotective genes [71] | Reduces cellular senescence; Enhances ECM synthesis [71] |
| Acute Lung Injury | Reduces inflammatory infiltration and cytokine levels [75] | Not specifically reported | Not specifically reported |
| Myocardial Infarction | Comprehensive cardioprotective effects; Promotes angiogenesis [69] | Not specifically reported | Improves ventricular function; Promotes cardiomyocyte regeneration [72] |
| Skin Regeneration | Promotes fibroblast proliferation and migration; Enhances tissue repair [70] | Promotes wound healing via TGF-β/Smad pathway [70] | Anti-fibrotic, proangiogenic, and neurotrophic properties [74] |
Immunomodulatory Effects
All three exosome types demonstrate significant immunomodulatory capabilities, though through potentially distinct mechanisms. UC-MSC exosomes are particularly noted for their strong immunomodulatory properties, including the ability to promote macrophage polarization toward an anti-inflammatory M2 phenotype [69]. Similarly, AD-MSC exosomes exhibit comprehensive immunomodulatory functions that support skin regeneration through immune regulation and anti-inflammatory responses [74]. BM-MSC exosomes have been shown to suppress macrophage activation and reduce proinflammatory macrophage infiltration, contributing to their therapeutic efficacy in inflammatory disease models [71].
Experimental Protocols
Exosome Isolation and Characterization
Standardized protocols for exosome isolation and characterization are essential for ensuring reproducible research outcomes and reliable therapeutic applications.
Protocol 1: Exosome Isolation via Ultracentrifugation
Materials Required:
- MSC-conditioned media (48-72 hour collection)
- Ultracentrifuge with fixed-angle or swinging-bucket rotor
- Polycarbonate bottles or thick-walled polypropylene tubes
- Phosphate-buffered saline (PBS), pH 7.4
- 0.22 μm filtration unit
Procedure:
- Centrifuge conditioned media at 2,000 × g for 30 minutes at 4°C to remove cells and large debris.
- Transfer supernatant to fresh tubes and centrifuge at 10,000 × g for 45 minutes at 4°C to remove apoptotic bodies and larger vesicles.
- Filter supernatant through 0.22 μm filter to remove remaining particles.
- Transfer filtered supernatant to ultracentrifuge tubes and centrifuge at 100,000 × g for 70 minutes at 4°C.
- Discard supernatant and resuspend pellet in PBS.
- Centrifuge again at 100,000 × g for 70 minutes at 4°C for a washing step.
- Resuspend final exosome pellet in PBS or appropriate buffer for storage at -80°C.
Protocol 2: Exosome Characterization
Nanoparticle Tracking Analysis (NTA):
- Dilute exosome preparation in PBS to achieve 20-100 particles per frame.
- Load sample into Nanosight or similar instrument.
- Perform three 60-second videos with camera level set to 13-16.
- Analyze data to determine particle size distribution and concentration [71] [70].
Transmission Electron Microscopy (TEM):
- Apply 10 μL of exosome suspension to Formvar/carbon-coated grid for 1 minute.
- Wick away excess liquid with filter paper.
- Stain with 2% uranyl acetate for 1 minute.
- Wick away excess stain and air dry.
- Image using TEM at 80-100 kV [71] [73].
Western Blot Analysis:
- Lyse exosomes in RIPA buffer.
- Separate proteins by SDS-PAGE and transfer to PVDF membrane.
- Probe with antibodies against exosomal markers (CD63, CD81, CD9, ALIX) and negative markers (Cytochrome C) [71] [73].
Figure 2: Experimental Workflow for Exosome Isolation and Characterization. Standardized protocols ensure reproducible preparation of high-quality exosomes for research and therapeutic applications.
In Vitro Functional Assays
Protocol 3: Anti-inflammatory Assessment
Materials:
- Chondrocytes or other relevant cell types
- IL-1β for inflammation induction
- Western blot equipment and reagents
- Antibodies for pp65, pp38, pJNK, pERK
Procedure:
- Pre-treat cells with MSC-derived exosomes (50-100 μg/mL) for 2 hours.
- Stimulate with IL-1β (10 ng/mL) for 30 minutes to 4 hours.
- Lyse cells and perform Western blot analysis for phosphorylated signaling proteins in NF-κB and MAPK pathways [71].
- Quantify band intensity to assess inhibition of inflammatory signaling activation.
Protocol 4: Wound Healing/Migration Assay
Materials:
- Human skin fibroblasts or endothelial cells
- Culture-inserts or scratching tools
- Time-lapse microscopy system
- Cell culture reagents
Procedure:
- Seed cells in culture-inserts or create a scratch wound in confluent monolayer.
- Treat with MSC-derived exosomes (20-100 μg/mL).
- Monitor cell migration every 6-12 hours using time-lapse microscopy.
- Quantify wound closure rate and compare between treatment groups [70].
Protocol 5: Angiogenesis/Tube Formation Assay
Materials:
- Human umbilical vein endothelial cells (HUVECs)
- Matrigel or other extracellular matrix substitutes
- Tissue culture plates
- Microscopy imaging system
Procedure:
- Plate HUVECs on Matrigel-coated plates.
- Treat with MSC-derived exosomes (50-100 μg/mL).
- Incubate for 4-18 hours at 37°C.
- Image tube networks and quantify number of branches, nodes, and total tube length [70].
Hydrogel Encapsulation Considerations
The encapsulation of MSC-derived exosomes in hydrogels represents an advanced delivery strategy for sustained release in wound healing applications. The selection of exosome source significantly influences hydrogel design parameters and therapeutic outcomes.
Exosome-Hydrogel Formulation Strategies
Three primary strategies exist for creating exosome-hydrogel composites:
- In situ hybrid cross-linking: Enables stimuli-triggered gelation and cavity-conformal encapsulation, particularly suitable for irregular wound geometries [17].
- Post-preloading cross-linking: Involves covalent exosome-polymer integration for enhanced stability and controlled release kinetics [17].
- Physical adsorption: Exploits hydrogel swelling dynamics for exosome incorporation and release [17].
Source-Specific Hydrogel Design Parameters
Table 3: Hydrogel Encapsulation Parameters by Exosome Source
| Parameter | UC-MSC Exosomes | BM-MSC Exosomes | AD-MSC Exosomes |
|---|---|---|---|
| Optimal Hydrogel Matrix | Hyaluronic acid, Chitosan-based [9] | Not specifically reported | PEG-based, Hyaluronic acid [17] |
| Release Kinetics | Sustained release over 72+ hours [17] | Not specifically reported | Programmable release based on hydrogel composition [17] |
| Therapeutic Concentration | 50-200 μg/mL in hydrogel matrix [70] | Not specifically reported | Varies by application [74] |
| Encapsulation Efficiency | >80% with proper hydrogel design [17] | Not specifically reported | >80% with proper hydrogel design [17] |
| Key Applications | Chronic wound healing, Diabetic ulcers [70] | Cartilage repair, Osteoarthritis [71] | Wound healing, Skin rejuvenation [74] |
Efficacy in Wound Models
Exosome-hydrogel composites have demonstrated remarkable efficacy in preclinical wound healing models. Hypoxia-pretreated ADSC-derived exosomes embedded in hydrogels increased the wound healing rate by approximately 30% and enhanced angiogenesis in rodent models [17]. These systems provide programmable release kinetics, such as 72-hour sustained VEGF delivery in vitro, and enable multifunctional regulation of the inflammatory microenvironment through antioxidant, immunomodulatory, and pro-angiogenic activities [17].
UC-MSC exosomes in hydrogel formulations significantly accelerate wound closure by reducing inflammation, stimulating angiogenesis, and promoting the formation of organized extracellular matrix [70]. Bioinformatic analyses suggest that ULK2, COL19A1, and IL6ST are potential key molecules involved in the regulation of wound repair by UC-MSC exosomes [70].
The Scientist's Toolkit: Essential Research Reagents
Table 4: Essential Research Reagents for Exosome Studies
| Reagent/Category | Specific Examples | Function/Application |
|---|---|---|
| Isolation Kits | Total Exosome Isolation Kit | Rapid isolation from conditioned media |
| Characterization Instruments | Nanosight NS300, ZetaView | Nanoparticle tracking analysis |
| Microscopy | Transmission Electron Microscope | Morphological validation |
| Cell Culture Reagents | MSC NutriStem XF Medium, Fetal Bovine Serum (exosome-free) | MSC expansion and exosome production |
| Hydrogel Materials | Hyaluronic acid, Chitosan, PEG | Exosome encapsulation and sustained release |
| Antibodies | Anti-CD63, Anti-CD81, Anti-CD9, Anti-ALIX | Exosome characterization via Western blot |
| Assay Kits | LIVE/DEAD Cell Imaging Kit, ApoLive-Glo Multiplex Assay | Viability and cytotoxicity assessment |
| Cytokines/Chemicals | IL-1β, LPS, Collagenase Type II | Disease modeling and cell isolation |
The comparative analysis of MSC-derived exosomes from umbilical cord, bone marrow, and adipose tissue reveals significant source-dependent variations in biological characteristics, functional efficacy, and therapeutic applications. UC-MSC exosomes demonstrate superior immunomodulatory and angiogenic properties, making them particularly suitable for wound healing applications when encapsulated in hydrogel delivery systems. BM-MSC exosomes exhibit strong chondroprotective and anti-inflammatory effects, while AD-MSC exosomes offer practical advantages of abundant tissue availability and potent tissue regenerative capacity. The selection of exosome source should be guided by the specific therapeutic goals, with UC-MSC exosomes representing the optimal choice for advanced hydrogel-based wound healing applications due to their comprehensive wound healing mechanisms and compatibility with sustained release systems. Future research should focus on optimizing source-specific hydrogel formulations to maximize the therapeutic potential of each exosome type in targeted clinical applications.
The field of regenerative medicine is increasingly pivoting from whole-cell therapies toward sophisticated cell-free approaches. Mesenchymal stem cell (MSC) transplantation has demonstrated therapeutic potential across numerous conditions, from ovarian insufficiency to cutaneous wounds, primarily through potent paracrine actions [76] [50]. Central to this paracrine activity are exosomes—nanosized extracellular vesicles (30–150 nm) that shuttle bioactive cargo between cells [3] [77]. This application note delineates the comparative efficacy of MSC-derived exosomes versus direct MSC transplantation, providing researchers with a structured analysis and actionable methodologies, contextualized within an advanced hydrogel-based delivery system for sustained wound release.
Comparative Therapeutic Efficacy: MSC Transplantation vs. MSC-Derived Exosomes
Direct comparisons in preclinical models reveal distinct profiles of efficacy, durability, and mechanism for whole cells versus their vesicular derivatives.
Table 1: Comparative Efficacy of MSC Transplantation vs. MSC-Derived Exosomes in a POI Mouse Model [76]
| Parameter | MSC-Treated Group | Exosome-Treated Group |
|---|---|---|
| First Breeding Cycle Pregnancy Rate | 60% to 100% | 30% to 50% |
| Second Breeding Cycle Pregnancy Rate | 60% to 80% | Infertile |
| Serum Hormone Level Restoration | Restored | Restored |
| Estrous Cycle Restoration | Restored | Restored |
| Therapeutic Durability | Long-term effect demonstrated | Short-term effect, requires repeat administration |
A landmark study comparing intravenous injection of MSCs versus equal amounts of MSC-derived exosomes in a chemotherapy-induced primary ovarian insufficiency (POI) mouse model yielded critical insights [76]. While both treatments were capable of restoring fertility in the first breeding round, the pregnancy rate was substantially higher in the MSC-treated group. The most striking difference emerged in the second round of breeding, where the MSC-treated group maintained a high pregnancy rate, but the exosome-treated group became infertile, indicating a lack of long-term durability with exosome therapy alone [76]. This suggests that while exosomes can effectively initiate recovery, MSCs may provide a more sustained therapeutic signal through continued residence and paracrine activity.
Beyond reproductive function, the therapeutic effects of both MSCs and their exosomes have been validated in other systems. In skin wound repair, MSC-exosomes promote angiogenesis, cell proliferation, collagen production, and inflammatory regulation [50]. Similarly, in models of retinal disease, small extracellular vesicles (sEVs) from bone marrow MSCs (BM-MSC-sEVs) demonstrated significant anti-apoptotic and proliferative effects on damaged retinal pigment epithelium cells, increasing cell viability from approximately 38% to over 54% after oxidative stress injury [78].
The Hydrogel Encapsulation Strategy for Enhanced Exosome Therapy
A significant challenge limiting the efficacy of free exosomes is their rapid clearance from the target site and limited retention [3] [50]. To overcome this, hydrogel-based delivery systems have been engineered to provide a protective, sustained-release platform.
Hydrogels are highly hydrophilic three-dimensional networks that swell in water without dissolving. Their porous structure allows for the physical encapsulation of exosomes and their controlled release over time, maintaining a high local concentration at the injury site [3]. For instance, chitosan hydrogels loaded with exosomes have shown excellent osteogenic properties in bone repair [3]. This strategy is particularly apt for wound healing, where the hydrogel can also serve as a protective barrier, creating a moist environment conducive to regeneration while steadily releasing therapeutically active exosomes [50].
Experimental Protocols for Key Applications
This protocol outlines the direct comparison of MSCs and exosomes in restoring ovarian function.
- POI Model Induction: Induce ovarian insufficiency in C57/BL6 mice via intraperitoneal injection of chemotherapeutic agents (e.g., cyclophosphamide and busulfan) using an established standard protocol.
- Therapeutic Administration: Post-chemotherapy, randomly assign mice to treatment groups. Resuspend MSCs (e.g., 1x10^5 cells) or an equivalent amount of exosomal particles (e.g., 1.5x10^8 particles, based on manufacturer yield data) in 100 µL of phosphate-buffered saline (PBS). Administer via retro-orbital intravenous injection.
- Efficacy Assessment:
- Molecular Analysis: At defined endpoints, harvest serum and ovarian tissue. Analyze serum hormone levels (FSH, AMH) via ELISA. Examine ovarian tissue for follicle count and apoptosis markers (e.g., TUNEL staining).
- Functional Fertility Assessment: In parallel, subject a separate cohort to breeding experiments. House treated female mice with proven fertile males and record pregnancy rates over multiple breeding cycles to assess both short-term and long-term functional restoration.
This protocol details the incorporation of exosomes into a hydrogel matrix for wound healing applications.
- Exosome Isolation and Characterization: Isolate MSC-exosomes from conditioned media using tangential flow filtration (TFF) or ultracentrifugation (UC). TFF generally offers higher particle yields and is more scalable [78]. Characterize particles by nanoparticle tracking analysis (NTA) for size/concentration, transmission electron microscopy (TEM) for morphology, and Western blotting for positive (CD9, CD63, TSG101) and negative (calnexin) markers [78].
- Hydrogel Preparation and Exosome Loading: Prepare a sterile, biocompatible hydrogel (e.g., chitosan). Prior to cross-linking, gently mix a concentrated suspension of characterized exosomes into the hydrogel precursor solution. Allow the mixture to cross-link under mild conditions to form the exosome-loaded hydrogel scaffold.
- In Vitro Release Kinetics and Bioactivity: Immerse the exosome-loaded hydrogel in PBS or simulated wound fluid at 37°C under gentle agitation. Collect release medium at predetermined time points and replace with fresh buffer. Quantify released exosome concentration via NTA or protein assay. Confirm the bioactivity of released exosomes using in vitro assays such as a cell proliferation assay (e.g., with fibroblasts or keratinocytes) to ensure retention of therapeutic function post-encapsulation and release.
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents for MSC-Exosome Hydrogel Research
| Reagent / Material | Function / Application | Key Considerations |
|---|---|---|
| Bone Marrow MSCs (BM-MSCs) | Gold-standard cellular source for exosome production; possess immunomodulatory and tissue-repair capabilities. | Verify expression of CD73, CD90, CD105; lack of CD34, CD45 [78]. |
| α-MEM Culture Medium | Cell culture medium for MSC expansion. | Yields higher cell proliferation and exosome yield compared to DMEM [78]. |
| Human Platelet Lysate (hPL) | Xeno-free supplement for MSC culture media. | Supports robust cell growth; preferred over fetal bovine serum for clinical translation [78]. |
| Tangential Flow Filtration (TFF) | Method for isolating and concentrating exosomes from conditioned media. | Provides higher particle yield and is more scalable than ultracentrifugation [78]. |
| Chitosan Hydrogel | A biocompatible and biodegradable natural polymer for exosome encapsulation. | Forms a sustained-release scaffold; promotes healing in wound applications [3] [50]. |
| Nanoparticle Tracking Analysis (NTA) | Instrumentation for determining exosome particle size and concentration. | Essential for quality control and dose standardization pre- and post-loading [78]. |
The choice between MSC transplantation and MSC-derived exosomes is not a simple substitution but a strategic decision. MSC-exosomes offer a cell-free, safer profile with reduced risks of immunogenicity and tumorigenicity, and are well-suited for off-the-shelf therapies [12] [50]. However, evidence suggests they may act with less durability than their cellular counterparts [76]. The integration of exosomes into hydrogel delivery systems represents a pivotal advancement, directly addressing the challenge of rapid clearance and unlocking the full therapeutic potential of exosomes for sustained tissue repair and regeneration. This combination approach holds particular promise for complex wound healing scenarios, where prolonged and localized treatment is paramount.
The management of chronic wounds, particularly diabetic foot ulcers, represents a significant and growing clinical challenge, characterized by delayed healing, disrupted immune response, and high economic burden [17] [79]. Within the evolving landscape of regenerative medicine, mesenchymal stem cell-derived exosomes (MSC-Exos) have emerged as promising acellular therapeutic agents. These nanoscale extracellular vesicles (typically 30-150 nm) facilitate intercellular communication by transferring bioactive cargo—including proteins, lipids, mRNA, and microRNA (miRNA)—to recipient cells, thereby modulating processes critical to wound repair such as inflammation, angiogenesis, and tissue regeneration [2] [67]. Compared to whole cell therapies, MSC-Exos offer distinct advantages: they exhibit lower immunogenicity, absent tumorigenic risk, enhanced safety profiles, and greater ease in storage and handling [23] [80].
However, a significant translational challenge has been the rapid clearance of free exosomes from wound sites when applied topically, which limits their therapeutic efficacy [80]. To address this limitation, hydrogel-based sustained delivery systems have been developed. These highly hydrophilic, three-dimensional polymer networks can be loaded with exosomes and applied directly to wounds, where they provide a moist environment, structural support, and, crucially, controlled release of the encapsulated vesicles [3] [17]. This combination of MSC-Exos with hydrogel technology represents a paradigm-shifting approach in wound care, synergizing the biological regenerative power of exosomes with the superior pharmacokinetics offered by advanced biomaterials [17].
Current Clinical Trial Landscape and Commercial Progress
The clinical translation of extracellular vesicle (EV)-based therapeutics, including exosome drugs, is advancing rapidly. Presently, there are more than 100 clinical studies globally evaluating both natural and engineered EV drugs for a range of conditions, including respiratory diseases, neurological disorders, severe acute inflammation, and tumors [2]. While the field is still young from a regulatory standpoint—with no specific technical guidelines yet issued by global drug regulatory authorities for EV-based drugs—the pipeline is expanding [2].
MSC-derived exosomes are among the most advanced EV-based therapeutics in development. They are positioned as intrinsic therapeutics, leveraging their native cargo to exert anti-inflammatory, immunomodulatory, and pro-regenerative effects, much like their parent cells [2]. Furthermore, engineered exosomes (eExo) are emerging as a highly favorable tool, where their cargo and surface properties can be tailored to enhance therapeutic efficacy and specificity for particular clinical indications, such as non-healing wounds and pathological scars [23].
Table 1: Key Characteristics of Natural vs. Engineered Exosome Therapeutics
| Feature | Natural Exosomes | Engineered Exosomes (eExo) |
|---|---|---|
| Definition | Isolated and purified from cell culture, biological fluid, or tissue without modification [2] | Cargo and/or surface properties are intentionally modified to enhance function [23] |
| Primary Advantage | Innate biological activity; relatively simpler production [2] | Enhanced therapeutic efficacy, specificity, and targeting potential [23] |
| Therapeutic Basis | Serves as intrinsic therapeutic agent [2] | Functions as enhanced intrinsic therapeutic or targeted delivery vehicle [23] [2] |
| Clinical Translation Stage | Closest to clinical translation and industrial production [2] | Greater long-term potential but development is more complex [2] |
Analysis of Translational Readiness
Translational readiness for hydrogel-encapsulated MSC exosomes can be evaluated across several key dimensions, from preclinical evidence to manufacturing and regulatory considerations.
Preclinical Efficacy and Mechanistic Evidence
Substantial preclinical data supports the therapeutic potential of MSC-Exo hydrogels. Studies consistently demonstrate accelerated wound closure, enhanced angiogenesis, promotion of re-epithelialization, and improved collagen remodeling in animal models of diabetic wounds [17] [79]. For instance, one study utilizing a hyaluronic acid-based injectable hydrogel to deliver MSC-Exos confirmed the system's ability to accelerate wound closure, enhance angiogenesis, and promote re-epithelialization in vivo [79]. Another study reported that hypoxia-pretreated adipose-derived stem cell (ADSC)-derived exosome-embedded hydrogels increased the wound healing rate by approximately 30% and enhanced angiogenesis in rodent models [17].
The mechanistic basis for this efficacy is multifaceted, involving the regulation of macrophage polarization (shifting from pro-inflammatory M1 to anti-inflammatory M2 phenotypes), promotion of vascular endothelial growth factor (VEGF) signaling for new blood vessel formation, and stimulation of keratinocyte and fibroblast proliferation and migration [67] [17]. These processes are orchestrated by the exosomal cargo, which modulates critical signaling pathways including PI3K/AKT, TGF-β/Smad, and Wnt/β-catenin in recipient cells [67].
Biomaterial Delivery Systems and Optimization
The choice of hydrogel is critical for achieving sustained, localized release of exosomes. A recent comparative study investigated a novel recombinant human collagen hydrogel (RHCMA) loaded with exosomes from three different MSC sources: human umbilical cord (ucMSC-exos), bone marrow (BMSC-exos), and adipose tissue (ADSC-exos) [19]. The study revealed that while all three systems promoted healing, ucMSC-exos@RHCMA demonstrated the best therapeutic performance, particularly in resolving inflammation, promoting angiogenesis, and improving collagen formation [19]. This highlights the importance of optimizing both the biomaterial scaffold and the exosome source for maximal clinical effect.
Table 2: Comparison of MSC Exosome Sources for Wound Healing Applications
| Exosome Source | Key Functional Strengths | Notable Cargo/Mechanisms |
|---|---|---|
| Adipose-Derived MSC (ADSC) | Promotes angiogenesis, supports blood vessel formation [19] | Rich in miRNA-125a, miRNA-31; targets IL-17RA via miR-192-5p to modulate scarring [3] |
| Bone Marrow-Derived MSC (BMSC) | Promotes cell growth and survival [19] | Contains anti-inflammatory factors (TSG-6, IL-10); promotes M1-to-M2 macrophage polarization via miR-23a-3p [3] |
| Umbilical Cord MSC (ucMSC) | Superior inflammatory resolution, angiogenesis, and collagen remodeling [19] | Helps regulate macrophages and reduce oxidative stress [19] |
Manufacturing, Quality Control, and Regulatory Pathways
The path to commercialization faces hurdles in manufacturing and regulation. A primary challenge is the large-scale production of exosomes in high purity and with consistent quality [2] [80]. The yield from standard isolation methods can be low, often resulting in less than 1 µg of exosomal protein from 1 ml of culture medium [80]. This is particularly challenging given that therapeutic doses in mouse models can range from 10–100 µg of exosomal protein, suggesting even larger scales would be needed for human trials [80].
Quality control is another critical area. The identity of exosomes is typically confirmed using a combination of techniques, including transmission electron microscopy for morphology, particle size analysis, and surface marker detection via Western blot or flow cytometry [3]. The regulatory pathway for EV-based drugs is still being defined. These products share properties with other advanced therapeutics, such as the targeted delivery of antibody-drug conjugates and the carrier function of gene therapies, but they also have unique characteristics [2]. As of now, no drug regulatory authority has issued specific technical guidelines for EV-based drugs, which adds uncertainty to the development process [2].
Detailed Experimental Protocols
To aid in the experimental validation of these therapies, below are detailed protocols for key processes in the development and evaluation of exosome-loaded hydrogels.
Protocol 1: Isolation and Characterization of MSC-Derived Exosomes
This protocol outlines the standard method for obtaining and validating exosomes from mesenchymal stem cell culture supernatants [19].
Step 1: Cell Culture and Conditioned Media Collection Culture MSCs (from umbilical cord, bone marrow, or adipose tissue) in appropriate media until 70-80% confluency. Replace the growth medium with exosome-depleted serum medium. Condition the cells for 48 hours. Collect the conditioned media and perform sequential centrifugation: first at 300 × g for 10 minutes to remove cells, then at 2,000 × g for 20 minutes to remove dead cells, and finally at 10,000 × g for 30 minutes to remove cell debris [81] [19].
Step 2: Exosome Isolation by Ultracentrifugation Filter the supernatant through a 0.22 µm filter. Ultracentrifuge the filtered supernatant at 100,000 × g for 70 minutes at 4°C. Carefully discard the supernatant and resuspend the resulting exosome pellet in a large volume of phosphate-buffered saline (PBS). Perform a second ultracentrifugation under the same conditions to wash the exosomes. Finally, resuspend the purified exosome pellet in a small volume of PBS (e.g., 100 µL) and store at -80°C [19].
Step 3: Exosome Characterization
- Transmission Electron Microscopy (TEM): Dilute the exosomes, adsorb onto a formvar-carbon coated grid, and stain with 2% uranyl acetate. Image using a TEM to confirm a cup-shaped, bilayer membrane morphology [19].
- Nanoparticle Tracking Analysis (NTA): Dilute the exosome sample appropriately and inject it into an NTA system (e.g., Malvern Nanosight) to determine the particle size distribution and concentration. The average size should be approximately 100-110 nm [19].
- Western Blot Analysis: Lyse the exosomes and separate proteins via SDS-PAGE. Transfer to a membrane and probe for positive exosomal markers (e.g., CD9, CD63, CD81, TSG101) and negative markers (e.g., Calnexin) to confirm purity [3].
Protocol 2: Fabrication of an Injectable Hyaluronic Acid (HA) Hydrogel Loaded with Exosomes
This protocol describes the creation of an in-situ crosslinking hydrogel for sustained exosome delivery, suitable for application to irregular wound beds [79].
Step 1: Preparation of Hydrogel Precursors Prepare a solution of hyaluronic acid modified with methacrylate groups (HA-MA) in PBS. In a separate vial, prepare a crosslinker solution (e.g., a dithiothreitol (DTT) solution). Keep both solutions on ice [79].
Step 2: Exosome Encapsulation and Gelation Mix the purified MSC exosomes (e.g., 100-500 µg of exosomal protein) with the chilled HA-MA precursor solution. Gently vortex to ensure a homogeneous mixture. Combine the exosome-HA-MA mixture with the crosslinker solution and mix thoroughly. The mixture will rapidly form a hydrogel at body temperature. For an injectable system, the liquid pre-gel solution can be drawn into a syringe and applied directly to the wound, where it will crosslink in situ [79].
Step 3: In Vitro Release Kinetics Profiling To characterize the release profile, load the exosomes with a lipophilic fluorescent dye (e.g., DiI or DiD) during step 2. Immerse a known volume of the formed exosome-loaded hydrogel in PBS at 37°C under gentle agitation. At predetermined time points, collect the release medium and measure the fluorescence intensity using a plate reader. Calculate the cumulative release percentage to generate a release profile, which typically shows an initial burst followed by a sustained release phase over several days [19].
Signaling Pathways and Therapeutic Mechanisms
The therapeutic effects of MSC-exosomes in wound healing are mediated through the modulation of several key signaling pathways in recipient cells. The following diagram synthesizes the primary mechanisms involved.
The Scientist's Toolkit: Essential Research Reagents and Materials
The table below catalogs key reagents and materials essential for conducting research on hydrogel-encapsulated MSC exosomes for wound healing.
Table 3: Essential Research Reagents and Materials
| Item Category | Specific Examples | Function/Application |
|---|---|---|
| MSC Sources | Umbilical Cord (ucMSCs), Bone Marrow (BMSCs), Adipose Tissue (ADSCs) [19] | Cellular source for exosome production; different sources confer varying functional properties [19]. |
| Hydrogel Polymers | Hyaluronic Acid (HA), Recombinant Human Collagen (RHC), Chitosan, Gelatin, Alginate [3] [79] [19] | Forms the 3D scaffold for exosome encapsulation, providing structural support and controlled release kinetics. |
| Crosslinkers | Methacrylate Anhydride (for modification), PEGDA, Dithiothreitol (DTT) [19] [80] | Induces gelation of polymer precursors to form the stable hydrogel network. |
| Exosome Isolation Kits | Ultracentrifugation kits, Precipitation-based kits (e.g., ExoQuick), Size-exclusion chromatography columns | Isolates and purifies exosomes from conditioned cell culture media. |
| Characterization Reagents | Antibodies against CD9, CD63, CD81, TSG101; Lipophilic dyes (DiI, DiD) for labeling; Uranyl acetate for TEM [19] | Enables the identification, quantification, and visualization of exosomes. |
| Cell Assay Kits | CCK-8 for proliferation, Transwell for migration, Tube formation for angiogenesis, ELISA for cytokines | Evaluates the biological activity and therapeutic efficacy of exosomes in vitro. |
The integration of MSC-derived exosomes with hydrogel delivery systems presents a compelling and innovative strategy for advanced wound care, with a rapidly evolving clinical trial landscape and a clear path toward translational readiness. The compelling preclinical data demonstrating enhanced wound closure, angiogenesis, and tissue regeneration provides a strong foundation for clinical advancement. The commercial viability of this approach will be determined by the successful navigation of key challenges, including the scaling up of GMP-compliant exosome production, the establishment of robust quality control metrics, and proactive engagement with regulatory agencies to define a clear approval pathway. Future progress will likely be fueled by the optimization of engineered exosomes for enhanced targeting and potency, the development of smarter, responsive hydrogels, and the execution of well-designed clinical trials that can definitively establish safety and efficacy in human patients.
Conclusion
The integration of MSC-derived exosomes with hydrogel technology represents a paradigm shift in regenerative wound care, moving beyond traditional cell-based therapies. The collective evidence confirms that hydrogel encapsulation successfully overcomes the major pharmacokinetic limitations of exosomes, providing a protective niche for their sustained and localized activity. This synergy not only enhances key wound healing processes—such as inflammation resolution, angiogenesis, and tissue regeneration—but also offers a safe, cell-free therapeutic alternative. Future efforts must focus on standardizing exosome isolation, optimizing hydrogel-exosome formulations for specific wound types, and conducting large-scale clinical trials. With its robust preclinical foundation, the hydrogel-exosome system holds immense promise for revolutionizing the treatment of chronic wounds and setting new standards in biomedical innovation.
References
- 1. Bioengineered MSC-derived exosomes in skin wound repair ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10009159/]
- 2. Extracellular vesicle-based drug overview [https://www.nature.com/articles/s41392-025-02312-w]
- 3. Different exosomes are loaded in hydrogels for the application ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11911322/]
- 4. The biogenesis and secretion of exosomes and multivesicular ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10363595/]
- 5. Current Knowledge on Exosome Biogenesis, Cargo ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9144303/]
- 6. Exosome biogenesis: machinery, regulation, and therapeutic ... [https://molecular-cancer.biomedcentral.com/articles/10.1186/s12943-022-01671-0]
- 7. Regulation of cargo selection in exosome biogenesis and ... [https://www.nature.com/articles/s12276-024-01209-y]
- 8. Latest developments in advanced exosome engineering ... [https://link.springer.com/article/10.1007/s44395-025-00023-3]
- 9. MSC-derived exosomes injectable hyaluronic acid ... [https://www.sciencedirect.com/science/article/pii/S0168365925006066]
- 10. Exosome-Based Drug Delivery: A Next-Generation Platform ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12567338/]
- 11. The tiny giants of regeneration: MSC-derived extracellular ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12310703/]
- 12. Mesenchymal Stem-Cell-Derived Exosomes and MicroRNAs [https://pmc.ncbi.nlm.nih.gov/articles/PMC12249468/]
- 13. The tiny giants of regeneration: MSC-derived extracellular ... [https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2025.1612589/full]
- 14. Exosomes: the future of acellular nanotherapeutics in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12443856/]
- 15. Mesenchymal stem cell-derived exosomes - PubMed Central [https://pmc.ncbi.nlm.nih.gov/articles/PMC8362559/]
- 16. Different exosomes are loaded in hydrogels for the ... [https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1545636/full]
- 17. Exo-hydrogel therapy: a revolutionary approach to managing ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12337418/]
- 18. A sprayable exosome-loaded hydrogel with controlled ... [https://pubmed.ncbi.nlm.nih.gov/40822934/]
- 19. Recombinant human collagen hydrogels with different stem ... [https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-025-03319-9]
- 20. A sprayable exosome-loaded hydrogel with controlled ... [https://www.sciencedirect.com/science/article/pii/S259000642500729X]
- 21. Researchers harnessing exosomes and hydrogels for ... [https://engineering.nyu.edu/news/researchers-harnessing-exosomes-and-hydrogels-advanced-diabetic-wound-healing]
- 22. Hydrogel scaffold encapsulating MSC-Exos and ZIF-8 ... [https://www.sciencedirect.com/science/article/pii/S2452199X2500386X]
- 23. Precision exosome engineering for enhanced wound healing ... [https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-025-06578-0]
- 24. Therapeutic Potential of Stem Cell-Derived Exosomes in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12383377/]
- 25. The utilization of exosomes in hydrogels - PubMed Central - NIH [https://pmc.ncbi.nlm.nih.gov/articles/PMC12486605/]
- 26. A novel hydrogel loaded with plant exosomes and stem ... [https://www.sciencedirect.com/science/article/pii/S2590006425003709]
- 27. Research Advances in Enhancing Wound Healing ... [https://www.sciencedirect.com/science/article/abs/pii/S0006291X25015037]
- 28. Exosome Isolation Protocols and Quantification Guidelines [https://www.sepscience.com/exosome-isolation-protocols-and-quantification-guidelines-12144]
- 29. A simplified and efficient method for isolating small ... [https://www.nature.com/articles/s41598-025-99822-y]
- 30. Labeling, isolation, and characterization of cell type-specific ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12505222/]
- 31. Transmission Electron Microscopy-based characterization of ... [https://biosignaling.biomedcentral.com/articles/10.1186/s12964-025-02383-w]
- 32. Advancements in hydrogels: A comprehensive review of ... [https://www.sciencedirect.com/science/article/abs/pii/S0141813025078274]
- 33. Advancements in Hydrogels: A Comprehensive Review of ... [https://www.mdpi.com/2073-4360/17/15/2026]
- 34. Hydrogel Encapsulation of Mesenchymal Stem Cells and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7827932/]
- 35. The Role of Natural Hydrogels in Enhancing Wound Healing [https://pmc.ncbi.nlm.nih.gov/articles/PMC12566826/]
- 36. Exosome-coated oxygen nanobubble-laden hydrogel ... [https://www.nature.com/articles/s41467-024-47696-5]
- 37. Hydrogel Encapsulation: Taking the Therapy of ... [https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2022.859927/full]
- 38. Artificial Intelligence-Assisted Conductive Hydrogel ... [https://link.springer.com/article/10.1007/s40820-025-01834-w]
- 39. Engineering Bioactive Self-Healing Antibacterial ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6332800/]
- 40. Exosome-Laden Hydrogels: A Novel Cell-free Strategy for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9011111/]
- 41. Exosome Mimetics-Loaded Hydrogel Accelerates Wound ... [https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2022.866505/full]
- 42. Hydrogels for Exosome Delivery in Biomedical Applications [https://www.mdpi.com/2310-2861/8/6/328]
- 43. Adipose-derived stem cell exosomes - Biomarker Research [https://biomarkerres.biomedcentral.com/articles/10.1186/s40364-025-00801-2]
- 44. Enhancing exosome stability and delivery with natural ... [https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-025-03603-8]
- 45. Exosome-based therapeutics in bone regeneration [https://stemcellres.biomedcentral.com/articles/10.1186/s13287-025-04686-8]
- 46. Enhancing angiogenesis through secretomes: Insights ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12167018/]
- 47. A novel hydrogel loaded with plant exosomes and stem ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12088786/]
- 48. Mesenchymal stem cell-derived exosomes: A novel and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9157601/]
- 49. Hydrogel Loaded with Extracellular Vesicles: An Emerging ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11280375/]
- 50. Bioengineered MSC-derived exosomes in skin wound ... [https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2023.1029671/full]
- 51. Advanced wound healing with chitosan hydrogels ... [https://www.frontiersin.org/journals/materials/articles/10.3389/fmats.2025.1573222/full]
- 52. Tailoring Therapy: Hydrogels as Tunable Platforms for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12469974/]
- 53. State-of-the-Art Insights into Hydrogel Innovations from ... [https://www.sciencedirect.com/science/article/abs/pii/S0924224425005473]
- 54. Foam-templated porous hydrogels: Tuning structure and ... [https://www.sciencedirect.com/science/article/abs/pii/S0032386125009887]
- 55. Succinamide ester-containing adhesive hydrogels with ... [https://www.sciencedirect.com/science/article/pii/S3050562325001679]
- 56. Hydrolytic degradation of PEG-based thiol–norbornene ... [https://pubs.rsc.org/en/content/articlehtml/2025/tb/d5tb01524c]
- 57. Recent Advances in Hydrogels for Tissue Engineering and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12470088/]
- 58. Hydrogel scaffold encapsulating MSC-Exos and ZIF-8 ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12628064/]
- 59. Preconditioning and Engineering Strategies for Improving the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9124131/]
- 60. preconditioning strategies for MSC-derived extracellular ... [https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2025.1509418/full]
- 61. Recombinant human collagen hydrogels with different stem... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11931813/]
- 62. Exo-hydrogel therapy: a revolutionary approach to managing ... [https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-025-03621-6]
- 63. Exosome-based therapeutics in bone regeneration [https://pmc.ncbi.nlm.nih.gov/articles/PMC12512519/]
- 64. Mesenchymal stem cell-derived extracellular vesicles [https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2025.1626996/full]
- 65. Mesenchymal stromal cell-laden hydrogels in tissue ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12464487/]
- 66. Exosomes in Diabetic Wound Healing: Mechanisms, ... [https://www.dovepress.com/exosomes-in-diabetic-wound-healing-mechanisms-applications-and-perspec-peer-reviewed-fulltext-article-DMSO]
- 67. Applications of mesenchymal stem cell-exosome ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11319788/]
- 68. Exosomes from adipose-derived stem cells accelerate ... [https://stemcellres.biomedcentral.com/articles/10.1186/s13287-025-04203-x]
- 69. Therapeutic potential of human umbilical cord mesenchymal ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12457780/]
- 70. Umbilical cord mesenchymal stem cell-derived exosomes ... [https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1641709/full]
- 71. Comparative Efficacy of Exosomes Derived from Different ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12193399/]
- 72. Adipose-derived stem cell exosomes: emerging roles and ... [https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2025.1637342/full]
- 73. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12299787/]
- 74. Therapeutic and Clinical Potential of Adipose-Derived ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12610433/]
- 75. Comparison of efficacy of exosomes derived from human ... [https://www.frontiersin.org/journals/pediatrics/articles/10.3389/fped.2025.1560915/full]
- 76. Comparison of the therapeutic effects between stem cells ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10283237/]
- 77. Stem cell derived exosome trilogy: an epic comparison of ... [https://stemcellres.biomedcentral.com/articles/10.1186/s13287-025-04440-0]
- 78. Comparison of production methods for mesenchymal stem ... [https://www.nature.com/articles/s41598-025-21218-9]
- 79. MSC-derived exosomes injectable hyaluronic acid ... [https://pubmed.ncbi.nlm.nih.gov/40581219/]
- 80. Sustained Delivery System for Stem Cell-Derived Exosomes [https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2019.01368/full]
- 81. Mesenchymal stem cell-derived exosomes: emerging ... [https://www.sciencedirect.com/science/article/pii/S2773041725000253]