Renal Cortex Injection of MSCs for AKI: Enhancing Paracrine Therapy and Overcoming Clinical Translation Barriers
Acute kidney injury (AKI) remains a significant clinical challenge with limited treatment options.
Renal Cortex Injection of MSCs for AKI: Enhancing Paracrine Therapy and Overcoming Clinical Translation Barriers
Abstract
Acute kidney injury (AKI) remains a significant clinical challenge with limited treatment options. Mesenchymal stem cells (MSCs) offer promising therapeutic potential primarily through their paracrine secretion of bioactive factors that promote tissue repair, modulate immune responses, and reduce inflammation. However, the clinical efficacy of MSC-based therapies is hampered by low cell retention and survival rates post-systemic delivery. This article explores renal cortex injection as a targeted local administration strategy to enhance MSC engraftment and paracrine activity in AKI settings. We examine the foundational mechanisms of MSC paracrine action, methodological considerations for renal cortex delivery, optimization strategies including preconditioning and 3D culture, and comparative efficacy against conventional administration routes. The synthesis of current evidence positions localized MSC delivery as a crucial advancement for realizing the full therapeutic potential of MSC-based AKI treatments.
Understanding AKI Pathophysiology and MSC Paracrine Mechanisms
Global Epidemiology of Acute Kidney Injury
Acute Kidney Injury represents a significant and growing clinical syndrome worldwide, characterized by a rapid decline in kidney function. Its epidemiology underscores a substantial public health burden.
Table 1: Global Epidemiology and Burden of Acute Kidney Injury
| Metric | Estimated Global Burden | Key Context |
|---|---|---|
| Annual Incidence | Approximately 13.3 million people per year [1] [2] | A leading cause of in-hospital mortality [3] |
| In-Hospital Mortality | As high as 62% [3] | |
| AKI in Critical Care | 5-6% of critical patients require renal replacement therapy [4] | Overall mortality in these critical patients is 58-62.6% [4] |
| Post-Transplant AKI | Incidence ranges from 17% to 95% (average ~40.7%) [5] | A common complication post-liver transplantation [5] |
| Progression to CKD | AKI is a major risk factor for new-onset CKD and accelerated progression [4] [6] |
The connection between AKI and Chronic Kidney Disease is particularly critical. AKI is now firmly established as a major risk factor for new-onset CKD and for accelerating the progression of pre-existing CKD [4] [6]. This progression is mediated through complex pathophysiological mechanisms, including maladaptive repair, persistent inflammation, and fibrotic pathways. The severity, duration, and frequency of AKI episodes are key determinants for the subsequent risk of CKD progression and mortality [4].
Experimental Protocols: Renal Cortex Injection of MSCs
The therapeutic potential of Mesenchymal Stem Cells for AKI is a cornerstone of regenerative nephrology. The following section provides a detailed protocol for the renal cortex injection of MSCs, a localized delivery method designed to maximize engraftment and paracrine effects at the injury site.
Protocol: Renal Subcapsular Injection of MSCs in a Rodent AKI Model
Objective: To administer MSCs directly into the renal cortex to enhance cell retention and survival in the injured kidney, thereby leveraging their paracrine therapeutic effects for AKI.
Materials and Reagents:
- MSCs: Human-derived MSCs (e.g., bone marrow, umbilical cord, or adipose tissue-derived), characterized by surface markers (CD73+, CD90+, CD105+, CD45-, CD34-, CD14-) [7] [4].
- Animal Model: Sprague-Dawley or Wistar rats (200-250 g), with AKI induced by ischemia-reperfusion, cisplatin, or gentamicin [3].
- Anesthesia: Isoflurane or ketamine/xylazine mixture.
- Surgical Tools: Sterile scalpel, forceps, retractors, and 6-0 silk suture.
- Injection Setup: Hamilton syringe (e.g., 50 µL) with a 30-gauge needle.
- Cell Preparation Solution: Phosphate-buffered saline (PBS) or sterile saline.
- Fluorescent Tracer: PKH26 red fluorescent cell linker dye for cell tracking [3].
Methodology:
- Preoperative Preparation:
- Culture and expand MSCs in standard conditions. Ensure they meet the minimal criteria for MSCs (plastic-adherent, specific surface marker expression, trilineage differentiation) [4].
- Cell Labeling and Harvest: Prior to injection, label MSCs with a fluorescent dye like PKH26 according to the manufacturer's protocol to enable subsequent tracking [3]. Harvest MSCs using trypsin-EDTA, wash with PBS, and resuspend in sterile saline at a concentration of 1–5 x 10^7 cells/mL. Keep the cell suspension on ice until use.
- Anesthetize the rat and shave the fur from the dorsal lumbar region. Sterilize the surgical site with betadine and ethanol.
Surgical Procedure:
- Make a 1.5–2 cm dorsal lateral incision to expose the left kidney.
- Gently mobilize the kidney and place it on sterile gauze.
- Using the Hamilton syringe with a 30-gauge needle, slowly inject 10–50 µL of the cell suspension (containing approximately 1–2 x 10^6 cells) into the upper and lower poles of the renal cortex, just beneath the renal capsule. A successful injection is indicated by a visible bleb.
- Hold the needle in place for 30 seconds after injection to prevent backflow.
- Return the kidney to the retroperitoneal space.
- Suture the muscle layer and skin separately.
Postoperative Care:
- Monitor animals until they fully recover from anesthesia.
- Administer analgesics (e.g., buprenorphine) for post-surgical pain.
- House animals under standard conditions with free access to food and water.
Analysis and Validation:
- Functional Assessment: Monitor renal function by measuring serum creatinine (Cr) and blood urea nitrogen (BUN) levels at baseline and days 1, 3, and 7 post-injection [3] [8].
- Histological Analysis: At the experimental endpoint, harvest kidney tissues. Process for histology (e.g., PAS staining) to evaluate tubular injury score, necrosis, and cast formation [3] [8].
- Cell Tracking: Analyze frozen kidney sections by fluorescence microscopy to detect PKH26-labeled MSCs, confirming their localization within the renal cortex [3].
- Mechanistic Studies: Perform TUNEL staining for apoptosis, immunohistochemistry for oxidative stress markers (e.g., 8-OHdG), and Western blot for apoptosis-related proteins (Bax, Bcl-2) and ER stress markers [3].
Diagram 1: Experimental workflow for renal cortex injection of MSCs in an AKI model.
Key Mechanisms of MSC Paracrine Therapy in AKI
The therapeutic benefits of MSCs are primarily mediated through their paracrine activity rather than direct differentiation and engraftment [9] [4]. The secreted factors and extracellular vesicles (EVs) act on multiple injury pathways in the damaged kidney.
Table 2: Key Paracrine Mechanisms of MSCs in AKI
| Mechanism of Action | Functional Impact | Key Mediators |
|---|---|---|
| Anti-Apoptosis | Reduces programmed cell death in tubular epithelial cells [3] [4]. | Growth factors, EVs carrying anti-apoptotic miRNAs; Upregulation of Bcl-2, downregulation of Bax and cleaved caspase [3]. |
| Anti-Inflammation & Immunomodulation | Modulates the immune response, reduces inflammatory cell infiltration [10] [7]. | Cytokines and EVs that increase regulatory T cells (Tregs); decrease pro-inflammatory cytokines [7]. |
| Anti-Oxidative Stress | Mitigates oxidative damage to renal cells [3]. | Enhancement of antioxidant defenses (e.g., GPx, catalase); reduction of oxidative markers (e.g., urinary 8-OHdG) [3]. |
| Pro-Angiogenic Effects | Promotes vascular regeneration and protects peritubular capillary density [10]. | Release of vascular endothelial growth factor (VEGF) and other angiogenic factors [10]. |
| Anti-Fibrotic | Inhibits progression to chronic kidney disease and fibrosis [8]. | Suppression of pro-fibrotic factors like TGF-β; reduction in extracellular matrix deposition [8]. |
Diagram 2: MSC paracrine mechanisms and therapeutic effects in AKI.
The Scientist's Toolkit: Research Reagent Solutions
This table outlines essential materials and reagents for investigating MSC-based paracrine therapy for AKI, with a focus on renal cortex injection protocols.
Table 3: Essential Research Reagents for MSC-based AKI Therapy
| Reagent / Material | Function / Application | Examples / Specifications |
|---|---|---|
| Mesenchymal Stem Cells (MSCs) | Core therapeutic agent; source of paracrine factors and EVs. | Bone marrow (BM-MSCs), umbilical cord (UC-MSCs), adipose (AD-MSCs). Must be CD105+, CD73+, CD90+, CD45-, CD34-, CD14- [7] [4]. |
| Cell Tracking Dyes | Labeling and visualization of administered MSCs to monitor homing and engraftment. | PKH26 (red fluorescent dye) [3]. |
| AKI Model Inducers | To establish experimental kidney injury models in rodents. | Gentamicin (70 mg/kg/day IP) [3], Cisplatin, Ischemia-Reperfusion Injury (IRI) models. |
| Renal Function Assay Kits | Quantitative assessment of kidney function impairment and recovery. | Commercial kits for Serum Creatinine (Cr) and Blood Urea Nitrogen (BUN) [3] [8]. |
| Antibodies for Mechanism | Detection of key proteins involved in AKI pathogenesis and repair. | Anti-Bax, Anti-Bcl-2 (apoptosis) [3]; Anti-Kim-1, Anti-NGAL (tubular injury) [8]; Anti-CD31, Anti-α-SMA (vascular and fibrosis). |
| ELISA Kits | Measurement of specific biomarkers in serum, urine, or tissue homogenates. | Urinary 8-OHdG for oxidative stress [3]; Cytokine kits for TNF-α, IL-6. |
| Extracellular Vesicle Isolation Kits | Isolation of EVs from MSC-conditioned media for mechanistic studies. | Based on differential ultracentrifugation, size-exclusion chromatography, or precipitation [4] [8]. |
| Hydrogel Polymers (for 3D Culture/Delivery) | Enhance MSC survival, retention, and paracrine function post-transplantation. | Natural polymers: Alginate, Chitosan, Agarose [2]. |
Risk Factors for AKI to CKD Progression
Understanding the factors that predispose patients to progression from AKI to CKD is critical for identifying at-risk populations for targeted therapies.
Table 4: Identified Risk Factors for Progression from AKI to CKD
| Risk Factor Category | Specific Factor | Impact (Odds Ratio or Risk Association) |
|---|---|---|
| Preoperative Comorbidities | Diabetes Mellitus [5] | OR 2.62 (95% CI 1.32–5.21) [5] |
| Hepatic Malignancy [5] | OR 1.95 (95% CI 1.06–3.57) [5] | |
| Elevated Preoperative Serum Creatinine [5] | OR 1.02 (per unit increase) (95% CI 1.01–1.03) [5] | |
| Postoperative Kidney Course | Transition from AKI to Acute Kidney Disease (AKD) [5] | OR 3.99 (95% CI 1.94–8.23) [5] |
| AKD Stages 2 and 3 [5] | OR 2.48 (95% CI 1.33–4.61) [5] | |
| eGFR < 60 mL/min/1.73 m² within 30 days post-op [5] | OR 3.03 (95% CI 1.70–5.40) [5] | |
| Protective Factors | Higher Preoperative Hematocrit [5] | Associated with reduced risk (OR 0.00; 95% CI 0.00–0.26) [5] |
| Recovery from AKD [5] | Associated with reduced risk (OR 0.49; 95% CI 0.27–0.86) [5] |
The Global AKI Burden and Limitations of Current Care
Acute Kidney Injury (AKI) represents a significant global health challenge, affecting approximately 13.3 million individuals annually and contributing to high rates of morbidity and mortality [1] [11]. Current management strategies primarily consist of supportive and preventive measures, with a notable absence of targeted pharmacological treatments that directly halt disease progression or promote tissue repair [11]. This therapeutic gap is particularly concerning given AKI's potential to advance to chronic kidney disease (CKD) and end-stage kidney disease, conditions that substantially diminish patients' quality of life and impose considerable financial strain on healthcare systems [11].
Mesenchymal Stem Cell Therapy: Mechanisms and Promise
Mesenchymal stem cells (MSCs) have emerged as a promising therapeutic intervention for AKI due to their multifaceted mechanisms of action, which extend beyond differentiation to include potent paracrine effects [11]. The therapeutic potential of MSCs is mediated through several key mechanisms:
- Paracrine Signaling: Secretion of bioactive molecules including cytokines, chemokines, growth factors, and extracellular vesicles that promote cell proliferation, inhibit apoptosis, enhance angiogenesis, and facilitate tissue repair [11].
- Immunomodulation: Modification of immune responses through anti-inflammatory capabilities and interaction with immune cells [11].
- Mitochondrial Transfer: Direct transfer of healthy mitochondria to injured renal cells via tunneling nanotubes, boosting cellular energy metabolism and contributing to tissue repair [11].
- Extracellular Vesicle-Mediated Effects: MSC-derived vesicles carrying therapeutic cargo that can modulate inflammatory pathways and promote recovery [12].
Table 1: Key Therapeutic Mechanisms of MSCs in AKI
| Mechanism | Biological Components | Functional Outcomes |
|---|---|---|
| Paracrine Signaling | Cytokines, chemokines, growth factors | Enhanced cell proliferation, reduced apoptosis, improved angiogenesis |
| Immunomodulation | Anti-inflammatory factors, regulatory enzymes | Reduced inflammation, altered immune cell polarization |
| Mitochondrial Transfer | Healthy mitochondria | Improved cellular energy metabolism, tissue repair |
| Vesicle-Mediated Effects | Extracellular vesicles (EVs), exosomes | Modulation of macrophage polarization, anti-inflammatory effects |
Critical Limitations in MSC-Based Therapies
Despite promising preclinical results, the clinical translation of MSC therapies faces significant challenges that limit their efficacy:
- Low Cell Retention and Survival: Transplanted MSCs exhibit poor retention and limited survival in the injured renal microenvironment, drastically reducing their therapeutic impact [11].
- Suboptimal Secretion of Therapeutic Factors: The hostile environment of injured renal tissues often leads to suboptimal production and secretion of the paracrine factors essential for renal repair [11].
- Homing Inefficiency: Systemically administered MSCs may fail to adequately migrate to and engraft within target tissues, with many cells becoming trapped in filtering organs like the lungs [11].
- Variable Patient Responses: Individual differences in disease etiology, severity, and patient physiology contribute to inconsistent therapeutic outcomes [13].
Strategic Approaches to Enhance MSC Efficacy
Preconditioning Strategies
Hypoxic Preconditioning Culturing MSCs under low oxygen conditions (1-5% O₂) prior to transplantation enhances their proliferation, survival, homing, differentiation, and paracrine activities [11]. This approach mimics the hypoxic microenvironment MSCs encounter post-transplantation and upregulates critical protective pathways:
- 1% O₂ preconditioning of human adipose-derived MSCs (hADMSCs) in rat IRI models demonstrated improvements in apoptosis reduction, enhanced anti-oxidative capacity, and increased vascularization [11].
- 5% O₂ preconditioning of human umbilical cord MSCs (hUCMSCs) increased expression of hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), integrins, and stromal-derived factor-1, ameliorating renal function in gentamicin-induced AKI models [11].
Chemical and Drug Preconditioning
- Chlorzoxazone (CZ): FDA-approved muscle relaxant that enhances MSC anti-inflammatory cytokine expression and strengthens immunosuppressive capacity without increasing immunogenicity [11].
- Atorvastatin (Ator): Statin medication with anti-apoptotic, antioxidant, and anti-inflammatory properties that synergistically enhances BMSC therapeutic effects [11].
Table 2: Preconditioning Strategies to Enhance MSC Efficacy
| Strategy Type | Specific Approach | Mechanistic Basis | Documented Outcomes |
|---|---|---|---|
| Physical Preconditioning | Hypoxic culture (1-5% O₂) | Upregulation of HIF-1α, CXCR4, enhanced paracrine function | Improved survival, angiogenesis, antioxidant capacity |
| Chemical Preconditioning | Chlorzoxazone | FOXO3 phosphorylation, anti-inflammatory phenotype | Reduced T-cell activation, decreased glomerular necrosis |
| Drug Preconditioning | Atorvastatin | Synergistic enhancement of inherent BMSC effects | Anti-apoptotic, antioxidant, anti-inflammatory benefits |
Advanced Delivery and Formulation Strategies
Three-Dimensional Culture Systems Advanced 3D culture methods, including hydrogels and spheroid formation, help recreate a more physiologically relevant microenvironment that enhances MSC functionality prior to transplantation [11].
Extracellular Vesicle-Based Approaches MSC-derived extracellular vesicles (MSC-EVs) offer numerous advantages over whole-cell therapies, including lower immunogenicity, reduced risk of tumor formation, and enhanced targetability [1] [12]. Specific findings include:
- Adipose-derived MSC-EVs (AMSC-EVs) attenuate AKI through modulation of the TXNIP-IKKα/NFκB signaling pathway in renal CX3CR1⁺ macrophages [12].
- EVs from induced pluripotent stem cells (iPSC-EVs) demonstrate superior efficacy compared to adipose-derived MSC-EVs in preserving mitochondrial integrity and regulating oxidative stress defense genes [1].
Genetic Modification Techniques Genetic engineering of MSCs to overexpress therapeutic factors can optimize their functionality and enhance secretory profiles, though this approach requires careful safety evaluation [11].
Detailed Experimental Protocol: Renal Cortex Injection of Preconditioned MSCs
MSC Preparation and Preconditioning
Materials Required:
- Primary human MSCs (bone marrow, adipose, or umbilical cord derived)
- Standard culture medium: DMEM/F12 supplemented with 10% FBS and 1% penicillin/streptomycin
- Hypoxia chamber or workstation
- Preconditioning agents: Chlorzoxazone (CZ) or Atorvastatin (Ator)
Procedure:
- Culture MSCs in standard conditions (37°C, 5% CO₂, 21% O₂) until 70-80% confluency.
- For hypoxic preconditioning: Transfer cells to hypoxia chamber (1-5% O₂, 5% CO₂, balanced N₂) for 24-48 hours prior to harvest.
- For chemical preconditioning: Treat MSCs with CZ (optimal concentration to be determined by dose-response studies) or Atorvastatin (5 μM) for 24 hours.
- Harvest cells using 0.25% trypsin-EDTA and resuspend in sterile PBS at concentration of 10,000-50,000 cells/μL for injection.
Surgical Procedure: Renal Cortex Injection in Rodent AKI Model
Animal Model: Male C57BL/6 mice (8-10 weeks old, 20-25g) AKI Induction: Cisplatin-induced model (single intraperitoneal injection, 20 mg/kg) or ischemia-reperfusion injury model (renal pedicle clamping, 30 minutes)
Injection Protocol:
- Anesthetize animal using isoflurane (3% induction, 1.5% maintenance) and place in right lateral decubitus position.
- Make a left flank incision and exteriorize the kidney using blunt dissection.
- Using a 30-gauge needle connected to a Hamilton syringe, slowly inject 10-20 μL of cell suspension (approximately 100,000-500,000 cells) at 2-3 sites within the renal cortex.
- Apply gentle pressure with sterile cotton tip for 30 seconds to prevent backflow.
- Return kidney to abdominal cavity and close incision in layers.
- Administer postoperative analgesia (buprenorphine, 0.1 mg/kg) and monitor until recovery.
Assessment and Analysis
Functional Assessment:
- Serum creatinine measurements at 24, 48, 72, and 96 hours post-AKI induction
- Blood urea nitrogen (BUN) levels
- Urine output measurement
Histological Analysis:
- Kidney tissue collection at 96 hours post-injury
- Paraffin embedding, sectioning (4μm), H&E staining
- Tubular injury scoring based on percentage of tubules showing epithelial necrosis, cast formation, or dilation
Molecular Analysis:
- Immunoblotting for TXNIP, IKKα, NFκB pathway components [12]
- Immunofluorescence for macrophage polarization markers (CD86 for M1, CD206 for M2)
- Cytokine profiling of renal tissue homogenates
Signaling Pathways and Experimental Workflow
MSC Therapeutic Mechanisms and Signaling Pathways
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for MSC-based AKI Research
| Reagent/Category | Specific Examples | Function/Application |
|---|---|---|
| MSC Sources | Bone marrow-derived MSCs, Adipose-derived MSCs, Umbilical cord MSCs | Primary therapeutic agents with multilineage differentiation potential |
| Preconditioning Agents | Chlorzoxazone (CZ), Atorvastatin, Hypoxic chambers | Enhance MSC survival, paracrine function, and therapeutic efficacy |
| Extracellular Vesicle Isolation | Differential centrifugation kits, Ultracentrifugation equipment, Size exclusion chromatography | Isolation and purification of MSC-derived vesicles for cell-free therapy |
| AKI Modeling Compounds | Cisplatin, Gentamicin, Ischemia-reperfusion surgical equipment | Induction of controlled kidney injury for experimental therapeutic testing |
| Molecular Analysis Tools | TXNIP antibodies, NFκB pathway inhibitors, CX3CR1 detection assays | Mechanism investigation and pathway modulation studies |
| Cell Tracking Methods | Fluorescent dyes (DiI, DiD), Lentiviral GFP labeling, Magnetic nanoparticles | Monitoring MSC migration, retention, and distribution post-delivery |
The development of targeted AKI interventions represents a critical unmet need in nephrology. While MSC-based therapies offer considerable promise through multiple mechanistic pathways, current limitations in cell retention, survival, and paracrine functionality must be addressed through strategic preconditioning, optimized delivery methods, and potentially cell-free alternatives such as extracellular vesicles. The renal cortex injection approach detailed in this protocol provides a targeted method for delivering enhanced MSCs directly to the site of injury, maximizing therapeutic potential while minimizing systemic losses. Future research directions should focus on optimizing preconditioning protocols, developing more efficient delivery systems, and conducting rigorous safety and efficacy studies to translate these promising approaches into clinical practice.
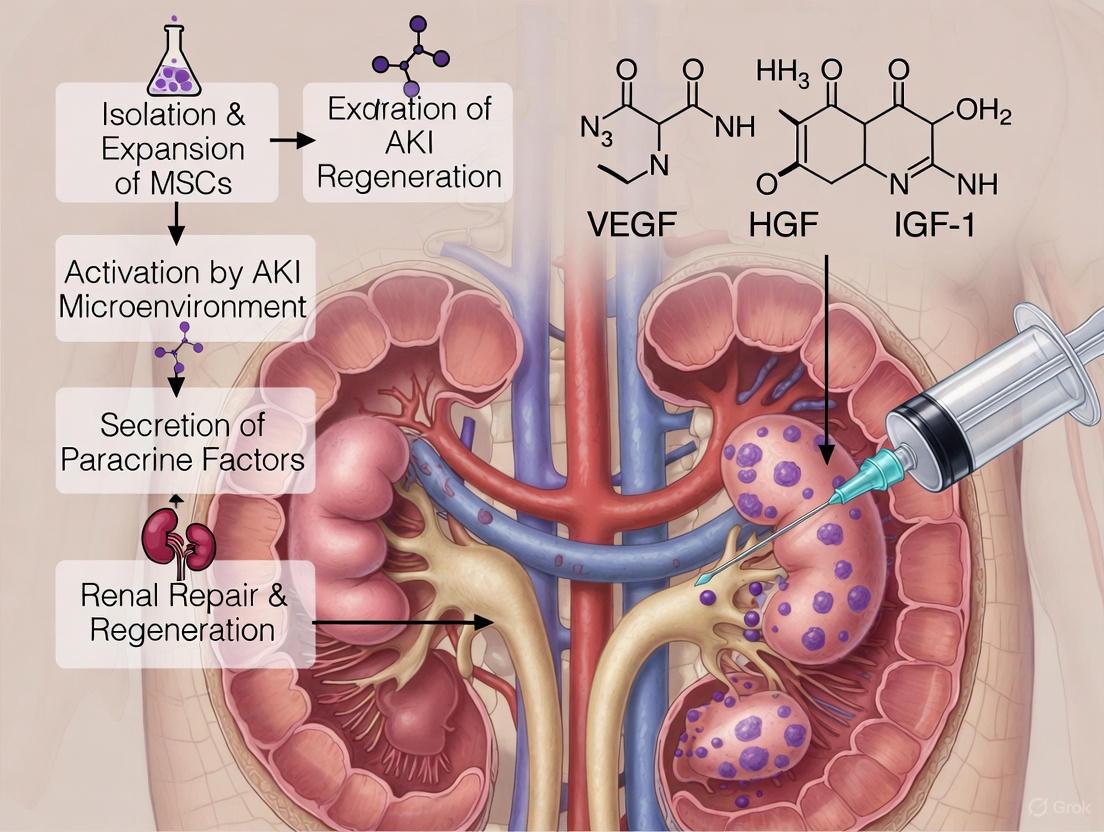
Acute Kidney Injury (AKI) represents a significant global health burden, with an estimated 13.3 million cases annually and strong associations with chronic kidney disease (CKD), end-stage renal disease (ESRD), and mortality [11] [14]. Current management remains predominantly supportive, creating an urgent need for targeted therapeutic interventions [11] [2]. Mesenchymal Stem/Stromal Cell (MSC)-based therapy has emerged as a promising regenerative strategy. Initially, the therapeutic potential of MSCs was attributed to their ability to differentiate and replace damaged renal cells [15]. However, accumulating preclinical and clinical evidence now indicates that the primary regenerative mechanism is paracrine action, mediated through the secretion of bioactive factors rather than direct cell replacement [16] [15] [17].
The MSC "secretome" comprises a complex mixture of soluble proteins (cytokines, chemokines, growth factors) and Extracellular Vesicles (EVs)—including exosomes and microvesicles—which deliver proteins, mRNAs, microRNAs, and lipids to recipient cells [16] [15] [17]. These factors collectively mitigate renal injury by inhibiting apoptosis, modulating immune responses, reducing oxidative stress, and promoting angiogenesis and tubular cell proliferation [16] [14] [18]. This application note details the composition of the MSC secretome, provides protocols for its study and application, and frames this within the context of direct renal cortex injection, a delivery method that enhances target engagement for AKI paracrine therapy research.
Composition of the MSC Secretome
The therapeutic efficacy of the MSC secretome is derived from its diverse cargo, which can be categorized into soluble factors and vesicular components.
Soluble Factors: Cytokines and Growth Factors
Proteomic analyses of MSC-conditioned media have identified a wide array of soluble factors central to renal repair. The table below summarizes the key functional categories and their principal constituents.
Table 1: Key Soluble Factors in the MSC Secretome and Their Roles in Renal Repair
| Factor Category | Key Constituents | Primary Functions in Renal Repair |
|---|---|---|
| Cytokines | IL-6, IL-10, IL-1RN (IL-1 receptor antagonist), LIF, TGF-β [15] [18] | Immunomodulation; shifting macrophages from pro-inflammatory M1 to anti-inflammatory M2 phenotype; suppressing T-cell activation [18]. |
| Growth Factors | HGF (Hepatocyte Growth Factor), VEGF (Vascular Endothelial Growth Factor), IGF-1 (Insulin-like Growth Factor 1), FGF-2 (Fibroblast Growth Factor 2), BMP-7 (Bone Morphogenetic Protein 7) [15] [14] [19] | Promoting tubular cell proliferation; inhibiting apoptosis; enhancing angiogenesis; attenuating fibrosis [15] [14]. |
| Chemokines | CCL2, CCL5, CXCL1, CXCL12 (SDF-1) [15] | Orchestrating cell migration and homing of progenitor cells to sites of injury. |
Extracellular Vesicles: Exosomes and Microvesicles
MSC-derived EVs are membrane-enclosed particles that act as primary mediators of intercellular communication by transferring bioactive molecules. Their cargo is selectively packaged and reflects the functional state of the parent MSCs [16] [17].
Table 2: Cargo and Function of MSC-Derived Extracellular Vesicles
| Cargo Type | Key Components | Documented Functions in AKI Models |
|---|---|---|
| mRNAs | mRNAs for transcription factors (e.g., POU3F1), angiogenesis (e.g., HGF, HES1), TGF-β signaling (e.g., TGFB1, TGFB3), and extracellular matrix remodeling (e.g., COL4A2) [16] [15]. | Transferred mRNAs can be translated in recipient cells, altering their phenotype and promoting repair processes. RNase treatment of EVs abolishes their therapeutic effects, underscoring the critical role of RNA cargo [15]. |
| MicroRNAs (miRNAs) | Various miRNAs (e.g., those targeting apoptotic and inflammatory pathways) [14]. | miRNAs inhibit the expression of target genes in recipient cells, leading to anti-apoptotic and anti-inflammatory effects. For instance, MSC-EVs upregulate anti-apoptotic genes (Bcl2, Bcl-xL) and downregulate caspases [14]. |
| Proteins | EV biogenesis markers (CD9, CD63, CD81), cytoskeletal proteins, and immunomodulatory factors [16] [15] [17]. | Proteins can directly activate signaling pathways in recipient cells. EVs from different MSC sources (adipose, bone marrow, umbilical cord) show heterogeneous protein content, influencing their restorative capacity [19]. |
The following diagram illustrates the biogenesis of these key secretome components and their subsequent actions on recipient renal cells.
Experimental Protocols for Secretome Analysis and Application
This section provides detailed methodologies for isolating the MSC secretome, establishing an in vitro AKI model, and evaluating therapeutic outcomes.
Protocol 1: Isolation and Characterization of MSC-Derived Extracellular Vesicles
Principle: EVs are isolated from MSC-conditioned medium via sequential ultracentrifugation to separate them from soluble factors and cellular debris, followed by characterization of their identity and concentration [12] [19].
Materials:
- Source: Human adipose-derived MSCs (AMSCs), bone marrow MSCs (BM-MSCs), or umbilical cord MSCs (UC-MSCs) [19].
- Culture Medium: DMEM/F12 or MEM-α supplemented with 10% EVs-depleted Fetal Bovine Serum (FBS) [12] [19].
- Reagents: Phosphate-Buffered Saline (PBS), Trypsin-EDTA.
- Equipment: Ultracentrifuge, fixed-angle rotor, Nanoparticle Tracking Analyzer, Transmission Electron Microscope, Western blot apparatus.
Procedure:
- Cell Culture and Supernatant Collection: Culture MSCs until 80% confluency. Replace medium with serum-free medium or medium containing EVs-depleted FBS. Collect conditioned medium after 24-48 hours.
- Differential Centrifugation:
- Centrifuge at 400 × g for 5-10 minutes to remove floating cells.
- Transfer supernatant and centrifuge at 10,000-20,000 × g for 20-30 minutes to remove apoptotic bodies and large debris.
- Filter the supernatant through a 0.22 µm filter.
- Ultracentrifugation: Transfer the filtered supernatant to ultracentrifuge tubes. Pellet EVs at 110,000 × g for 70-90 minutes at 4°C. Carefully discard the supernatant.
- Washing: Resuspend the EV pellet in a large volume of PBS. Perform a second ultracentrifugation under the same conditions to remove contaminating soluble proteins.
- Final Resuspension: Resuspend the final, clean EV pellet in a small volume of PBS and store at -80°C.
- Characterization:
- Nanoparticle Tracking Analysis (NTA): Dilute EVs in PBS and inject into the NTA to determine particle size distribution and concentration [12].
- Transmission Electron Microscopy (TEM): Apply EVs to a formvar-carbon coated grid, negative stain with uranyl acetate, and image to confirm classic "cup-shaped" morphology [16] [12].
- Immunoblotting: Confirm the presence of EV-positive markers (CD9, CD63, CD81) and the absence of negative markers (e.g., Calnexin) [12].
Protocol 2: In Vitro Modeling of AKI and Secretome Treatment
Principle: Chemically induce ischemic injury in human proximal tubule epithelial cells to model AKI and assess the restorative effects of the MSC secretome [19].
Materials:
- Cell Line: Human proximal tubule epithelial cells.
- Chemicals: Antimycin A (complex III inhibitor), 2-deoxy-D-glucose (glycolysis inhibitor) [19].
- Treatment: MSC-conditioned medium or characterized EVs.
Procedure:
- Induction of Ischemic Injury: Culture proximal tubule cells until 70-80% confluency. Induce injury by treating cells with 10 nM Antimycin A and 20 mM 2-deoxy-D-glucose in serum-free medium for 24 hours. Maintain under normoxic (21% O₂) or hypoxic (1% O₂) conditions to better mimic ischemia [19].
- Treatment Application: After 24 hours, wash cells with HBSS to remove chemical inhibitors. Treat injured cells with:
- MSC-conditioned medium, or
- Isolated EVs (dose: bioproduct equivalent from 2 MSCs per tubule cell) [19].
- Include controls: sham (healthy), vehicle (serum-free medium), and reperfusion (serum-containing medium).
- Incubation: Incubate cells with the secretome treatment for 24 hours.
Protocol 3: Assessing Therapeutic Efficacy
Principle: Quantify the restoration of cellular health and function post-treatment using a suite of biochemical and metabolic assays.
Key Assays:
- Cell Metabolic Activity: Measure using PrestoBlue or MTT assay. Expect a significant increase in metabolic activity in treated groups versus injured controls [19].
- ATP Production: Quantify using a luciferase-based assay. Successful treatment should restore ATP levels, a key indicator of bioenergetic recovery [19].
- Apoptosis Assay: Perform TUNEL staining or caspase-3/7 activity assay. MSC secretome treatment should significantly reduce apoptotic activity [14].
- Metabolomic Analysis: Utilize LC-MS or GC-MS to profile cellular metabolites. Look for increased levels of glycolysis intermediates and antioxidant metabolites (e.g., glutathione) post-treatment, indicating metabolic restoration and reduced oxidative stress [19].
- Macrophage Polarization Assay: Co-culture treated injured cells with monocytes or use in vivo models. Flow cytometry for M2 markers (CD206) vs. M1 markers (CD86) can demonstrate the immunomodulatory effect of the secretome, a key mechanism in vivo [12].
The Scientist's Toolkit: Essential Research Reagents
The table below lists critical reagents and their applications for researching the MSC secretome in renal repair.
Table 3: Key Research Reagents for MSC Secretome Studies
| Research Reagent / Tool | Function and Application | Example Usage in Protocol |
|---|---|---|
| EVs-depleted FBS | Essential for cell culture during EV production. Standard FBS contains bovine EVs that contaminate preparations. EVs are removed via ultracentrifugation. | Used in Protocol 1 for conditioning media to ensure isolation of pure, human MSC-derived EVs [12] [19]. |
| CD9, CD63, CD81 Antibodies | Surface markers characteristic of exosomes and other EVs. Used for immunoblotting or flow cytometry to confirm EV identity and purity. | Used in Protocol 1, Step 6 (Immunoblotting) for the characterization of isolated EVs [12] [17]. |
| Antimycin A & 2-deoxy-D-glucose | Chemical inducers of in vitro ischemia. They inhibit mitochondrial respiration and glycolysis, respectively, mimicking the bioenergetic collapse in AKI. | Used in Protocol 2, Step 1 to induce a reproducible and controlled ischemic injury in proximal tubule cells [19]. |
| PrestoBlue / MTT Reagent | Cell-permeable dyes used as indicators of cell viability and metabolic activity. The conversion rate correlates with the number of viable cells. | Used in Protocol 3 to quantitatively assess the recovery of cellular health following secretome treatment [19]. |
| TXNIP Antibodies / siRNA | Tools to investigate a key molecular mechanism. TXNIP is a protein that regulates the NF-κB signaling cascade and macrophage polarization. | Used in mechanistic studies to validate the TXNIP-IKKα/NF-κB pathway, through which AMSC-EVs promote M2 macrophage polarization [12]. |
Research Context: Renal Cortex Injection for AKI Therapy
The route of administration is a critical factor for the success of MSC secretome-based therapies. While intravenous (IV) infusion is common, it results in significant entrapment of cells or EVs in the lungs and liver, reducing the fraction that reaches the kidneys [2]. Direct renal cortex injection is an advanced delivery method that offers distinct advantages for preclinical research and potential clinical translation.
This targeted approach involves the precise inoculation of MSCs or their secretome (e.g., concentrated EVs) directly into the renal parenchyma. It is typically performed under ultrasound or surgical guidance to ensure accuracy [2]. The primary advantage is the bypass of systemic filtration, enabling a high local concentration of therapeutic agents to be delivered directly to the site of injury. This maximizes paracrine signaling and minimizes off-target distribution [2]. Studies have shown that local injection increases cell engraftment and enhances functional recovery in animal models of AKI, without reported significant safety concerns like renal embolism when performed appropriately [2].
For researchers, this model is highly relevant for investigating the localized paracrine effects of MSCs. It allows for the precise tracking of EV uptake, the analysis of local immune modulation (e.g., CX3CR1+ macrophage polarization within the kidney), and the assessment of direct tubular repair mechanisms [12]. The workflow below outlines the key stages of a research project utilizing renal cortex injection.
The MSC secretome, through its rich composition of cytokines, growth factors, and EVs, represents a powerful, cell-free therapeutic tool for renal repair, effectively attenuating apoptosis, inflammation, and bioenergetic failure in AKI. The efficacy of this paracrine action can be significantly amplified by employing direct renal cortex injection, which ensures optimal delivery to the target tissue. The protocols and tools detailed in this application note provide a foundational framework for researchers to rigorously isolate, characterize, and test the MSC secretome, advancing the translational path towards a novel and effective therapy for Acute Kidney Injury.
Macrophage Polarization: Mechanisms and Experimental Evidence
Mesenchymal Stem Cells (MSCs) promote the polarization of pro-inflammatory M1 macrophages towards the anti-inflammatory, reparative M2 phenotype through multiple paracrine signaling pathways. This immunomodulatory action is a cornerstone of their therapeutic effect in Acute Kidney Injury (AKI).
Key Signaling Pathways in Macrophage Polarization
The table below summarizes the primary mechanisms by which MSCs and their derivatives modulate macrophage polarization, as evidenced in preclinical AKI models.
Table 1: Mechanisms of MSC-Mediated Macrophage Polarization in AKI
| Mechanism / Signal | MSC Source | Experimental Model | Key Finding | Reference |
|---|---|---|---|---|
| miR-146a-5p inhibits TRAF6/STAT1 | Umbilical Cord (hUMSCs-Exo) | Diabetic Kidney Disease (DKD) Model | Facilitated M1-to-M2 transition, reducing inflammation and improving renal function. | [20] |
| miR-486-5p activates PI3K/Akt pathway | Umbilical Cord (hUMSCs-Exo) | DKD Model | Promoted M2 polarization, alleviating tubular injury and glomerulosclerosis. | [20] |
| TFEB-mediated autophagy | Bone Marrow (BMSCs) | Diabetic Nephropathy Model | Promoted M2 polarization, inhibiting inflammation and mesangial matrix expansion. | [20] |
| TXNIP-IKKα/NF-κB suppression | Adipose Tissue (AMSC-EVs) | Cisplatin-induced AKI Mouse Model | Promoted polarization of renal CX3CR1+ macrophages to reparative M2 type. | [12] |
| Inflammatory Priming (IL-1β, TNF-α, IL-17) | Wharton's Jelly (WJ-MSCs) | In vitro macrophage co-culture | Primed MSC secretome significantly enhanced ability to polarize macrophages to M2 phenotype. | [21] |
Detailed Experimental Protocol: Evaluating Macrophage PolarizationIn Vivo
The following protocol is adapted from studies investigating MSC therapy in murine AKI models [12] [20].
Objective: To assess the effect of renal cortex-injected MSCs on macrophage polarization in kidney tissue of mice with cisplatin-induced AKI.
Materials:
- Animal Model: C57BL/6 mice (8-10 weeks old)
- AKI Induction Agent: Cisplatin (e.g., from MedChemExpress, HY-17394)
- MSCs: Human adipose-derived MSCs (AMSCs) or bone marrow-derived MSCs (BMSCs), passages 3-8.
- Key Reagents:
- Flow Cytometry Antibodies: Anti-mouse F4/80 (APC), CD86 (FITC) for M1 markers, CD206 (PE) for M2 markers.
- ELISA Kits: Mouse IL-1β, TNF-α, IL-10, TGF-β.
- RNA Isolation Kit and qPCR reagents.
- Primary Antibodies for IHC: iNOS (M1), Arginae-1 (M2).
Procedure:
- AKI Induction and MSC Administration:
- Induce AKI in mice via a single intraperitoneal (i.p.) injection of cisplatin (10-12 mg/kg).
- At 24 hours post-cisplatin injection, randomly assign mice to treatment groups.
- Under anesthesia, administer MSCs (e.g., 1-2x10^5 cells in 50µL PBS) via renal cortex injection into both kidneys using a 30-gauge insulin syringe. Control groups receive an equivalent volume of PBS.
- Tissue Collection:
- At 96 hours post-injury, euthanize mice and perfuse kidneys with cold PBS.
- Collect kidney tissues for:
- Flow Cytometry: Dispase/collagenase-digested single-cell suspension.
- RNA/Protein Analysis: Snap-frozen in liquid nitrogen.
- Histology: Fixed in 4% paraformaldehyde and embedded in paraffin.
- Flow Cytometric Analysis:
- Prepare a single-cell suspension from kidney tissue.
- Stain cells with surface marker antibodies: F4/80 (macrophages), CD86 (M1), and CD206 (M2).
- Analyze using flow cytometry. Calculate the ratio of F4/80+CD206+ (M2) to F4/80+CD86+ (M1) cells.
- Cytokine Profiling:
- Homogenize kidney tissue and quantify supernatant levels of pro-inflammatory (IL-1β, TNF-α) and anti-inflammatory (IL-10, TGF-β) cytokines by ELISA.
- Gene Expression Analysis (qPCR):
- Extract total RNA from kidney tissue and synthesize cDNA.
- Perform qPCR to analyze expression of M1 markers (iNOS, CD86) and M2 markers (Arg-1, CD206, Ym-1). Normalize to GAPDH.
- Immunohistochemistry (IHC):
- Perform IHC on kidney sections using antibodies against iNOS and Arginae-1.
- Quantify positive cell infiltration in the renal tubulointerstitial area.
Diagram 1: MSC-Mediated Macrophage Polarization Signaling Pathways. MSCs and their extracellular vesicles (EVs) promote M2 macrophage polarization via multiple microRNA and protein-level pathways, suppressing key pro-inflammatory signaling cascades.
T-cell Suppression: Mechanisms and Experimental Protocols
MSCs suppress the activation and proliferation of T-cells, a major driver of inflammation in AKI, through both cell-to-cell contact and the release of soluble factors.
Key Mechanisms of T-cell Suppression
The primary mechanisms of T-cell suppression by MSCs include:
- Soluble Factor Secretion: MSCs secrete immunomodulatory factors such as Prostaglandin E2 (PGE2), Indoleamine 2,3-dioxygenase (IDO), Transforming Growth Factor-β (TGF-β), and Human Leukocyte Antigen-G5 (HLA-G5) [22]. These molecules directly inhibit T-cell proliferation and effector functions.
- Metabolic Disruption: The enzyme IDO catalyzes the conversion of tryptophan into kynurenines. Depleting local tryptophan and accumulating kynurenines induces cell cycle arrest and apoptosis in activated T-cells [22].
- Chemical Preconditioning: Preconditioning MSCs with drugs like Chlorzoxazone (CZ), an FDA-approved muscle relaxant, enhances their immunosuppressive capacity. CZ promotes FOXO3 phosphorylation, boosting the expression of IDO and other anti-inflammatory cytokines, which more effectively attenuates renal inflammation in AKI models [11].
Detailed Experimental Protocol: Assessing T-cell Proliferation and ActivationIn Vitro
This protocol is used to evaluate the immunomodulatory potency of MSCs, particularly after preconditioning strategies [11] [22].
Objective: To measure the suppression of T-cell proliferation and activation by MSC-conditioned medium (MSC-CM) or via direct co-culture.
Materials:
- MSCs: Bone marrow or umbilical cord-derived MSCs.
- Peripheral Blood Mononuclear Cells (PBMCs): Isolated from human blood.
- T-cell Mitogen: Phytohemagglutinin (PHA) or Anti-CD3/CD28 antibodies.
- Cell Tracking Dye: CFSE (Carboxyfluorescein succinimidyl ester).
- Key Reagents:
- Cell Culture Media: RPMI-1640 for PBMCs, DMEM/F12 for MSCs.
- Fetal Bovine Serum (FBS), Penicillin/Streptomycin.
- Preconditioning Agent: Chlorzoxazone (CZ, e.g., from Sigma-Aldrich).
- Flow Cytometry Antibodies: Anti-human CD3 (APC), CD4 (FITC), CD8 (PerCP), CD25 (PE), CD69 (PE).
- ELISA Kits: Human IFN-γ, IL-2.
Procedure:
- Generation of MSC-Conditioned Medium (MSC-CM):
- Culture MSCs until 70-80% confluency.
- For preconditioning, treat MSCs with a non-cytotoxic dose of CZ (e.g., 50 µM) for 24-48 hours [11].
- Replace medium with fresh, serum-free medium for another 24-48 hours.
- Collect the supernatant, centrifuge to remove cell debris, and use as MSC-CM. Store at -80°C.
- T-cell Proliferation Assay (CFSE Dilution):
- Isolate PBMCs and label with CFSE according to manufacturer's instructions.
- Activate CFSE-labeled PBMCs (1-2x10^5 cells/well) with PHA (5 µg/mL) in a 96-well plate.
- Co-culture activated PBMCs with:
- Direct Co-culture: Various ratios of MSCs (e.g., 1:10, MSC:PBMC).
- Indirect Culture: Using MSC-CM (e.g., 50% v/v in PBMC media).
- Include controls: Non-activated PBMCs and PHA-activated PBMCs alone.
- After 3-5 days, harvest cells and stain with anti-CD3 antibody.
- Analyze CFSE fluorescence intensity in CD3+ T-cells by flow cytometry. Reduced CFSE fluorescence indicates cell division.
- T-cell Activation Marker Analysis:
- Co-culture PBMCs with MSCs or MSC-CM as described above.
- After 24-48 hours, harvest cells and stain with anti-CD3, anti-CD25 (IL-2 receptor α-chain), and anti-CD69 (early activation marker) antibodies.
- Analyze by flow cytometry. The percentage of CD3+ T-cells expressing CD25 and CD69 reflects the level of T-cell activation.
- Cytokine Analysis:
- Collect supernatant from the co-cultures after 48-72 hours.
- Measure levels of T-cell-derived pro-inflammatory cytokines (e.g., IFN-γ, IL-2) using ELISA.
Table 2: The Scientist's Toolkit - Key Research Reagents for MSC Immunomodulation Studies
| Reagent / Material | Function / Application | Example Use Case |
|---|---|---|
| Chlorzoxazone (CZ) | Chemical preconditioning of MSCs to enhance anti-inflammatory phenotype and IDO expression. | Boosting MSC efficacy in suppressing T-cell proliferation in vitro and in AKI models. [11] |
| CFSE (Cell Tracking Dye) | Fluorescent dye that dilutes with each cell division, allowing quantification of T-cell proliferation by flow cytometry. | Tracking the suppressive effect of MSC-CM on mitogen-activated T-cell divisions. |
| Recombinant Human Cytokines (IL-1β, TNF-α) | Inflammatory priming of MSCs to enhance their immunomodulatory secretome. | Priming MSCs to increase secretion of TSG-6 and IL-6, enhancing macrophage polarization capacity. [21] |
| Flow Cytometry Antibodies (F4/80, CD86, CD206, CD3, CD25) | Cell surface marker identification for phenotyping macrophages (M1/M2) and activated T-cells. | Quantifying shifts in macrophage populations and T-cell activation status in co-cultures or kidney tissue. [12] [20] |
| ELISA Kits (IFN-γ, IL-2, IL-1β, IL-10) | Quantification of soluble inflammatory and anti-inflammatory cytokines in culture supernatant or tissue homogenates. | Profiling the inflammatory microenvironment to confirm immunomodulatory action of MSCs. |
Diagram 2: Experimental Workflow for Analyzing MSC-Mediated T-cell Suppression. The process involves preconditioning MSCs to enhance their potency, followed by co-culture with activated T-cells and measurement of suppression outcomes through various assays.
The immunomodulatory actions of MSCs, particularly their capacity to polarize macrophages towards an M2 phenotype and suppress T-cell activation, form a robust mechanistic basis for their therapeutic application in AKI. Optimizing these effects through strategies like inflammatory priming, genetic modification, and precise delivery methods such as renal cortex injection is a critical focus of current research, holding significant promise for the development of effective cell-based therapies for inflammatory kidney diseases.
Acute kidney injury (AKI) is a prevalent clinical syndrome characterized by a rapid decline in renal function, associated with high morbidity and mortality, and currently lacks highly effective targeted therapies [23]. The kidney, particularly its proximal tubules, is an organ with high energy demands, consuming approximately 7% of the body's daily ATP expenditure to perform its filtration and reabsorption functions [23]. This high metabolic requirement makes it exceptionally susceptible to mitochondrial dysfunction, which is now recognized as a central pathophysiological event in AKI triggered by various insults, including ischemia-reperfusion (I/R) injury, sepsis, and nephrotoxic agents like cisplatin [23] [24].
Mitochondrial dysfunction in AKI manifests through multiple interconnected processes: impaired mitochondrial biogenesis (the generation of new mitochondria), disrupted mitochondrial dynamics (the balance between fission and fusion), and defective mitophagy (the selective removal of damaged mitochondria) [23]. These alterations lead to ultrastructural changes such as mitochondrial swelling and fragmentation, decreased ATP production, elevated oxidative stress due to reactive oxygen species (ROS) overproduction, and initiation of apoptotic pathways—collectively culminating in renal tubular epithelial cell death and loss of renal function [23] [24].
Mesenchymal stem cells (MSCs) have emerged as promising therapeutic candidates for AKI, not only through their paracrine activities but also via a more novel mechanism: intercellular mitochondrial transfer [25]. This process involves the delivery of functional mitochondria from MSCs to injured renal cells, thereby restoring cellular bioenergetics and promoting survival. This Application Note details the protocols and mechanistic insights for harnessing mitochondrial transfer as a therapeutic strategy within the broader context of renal cortex-directed MSC therapy for AKI.
Mechanisms of Mitochondrial Transfer from MSCs to Injured Renal Cells
MSCs utilize several distinct yet potentially overlapping mechanisms to transfer mitochondria to recipient cells, each with unique structural and functional characteristics.
Table 1: Mechanisms of Mitochondrial Transfer in AKI
| Transfer Mechanism | Key Mediating Structures/Components | Process Description | Functional Significance in AKI |
|---|---|---|---|
| Tunneling Nanotubes (TNTs) | Actin-based cytoplasmic bridges [25] [26] | Formation of thin, open-ended channels enabling direct transfer of organelles, including intact mitochondria, between cells. | Direct, targeted delivery of healthy mitochondria to stressed tubular cells, restoring ATP production [25]. |
| Extracellular Vesicles (EVs) | Exosomes, microvesicles [25] | Packaging of mitochondria or mitochondrial components into membrane-bound vesicles released into extracellular space for uptake by recipient cells. | Paracrine delivery of mitochondrial material; can be engineered for enhanced therapeutic delivery [12] [27]. |
| Direct Cell-Cell Contact | Gap junctions, adhesion molecules [26] | Close membrane apposition facilitating transfer of cellular contents, potentially including small mitochondrial fragments or components. | Coordination of metabolic responses in adjacent cells within the tubular epithelium [25]. |
The transfer process is typically initiated by distress signals from injured renal tubular cells, such as elevated reactive oxygen species (ROS) or the release of ADP [25] [28]. MSCs sense these signals and respond by initiating the formation of TNTs or packaging mitochondria into EVs. The efficiency of this process can be enhanced by engineering MSCs to overexpress Miro1, a protein critical for mitochondrial trafficking along the cytoskeleton [25].
Diagram 1: Mitochondrial Transfer Pathway in AKI. This diagram illustrates the sequence from initial kidney injury to cellular recovery via different mitochondrial transfer mechanisms.
Quantitative Data on Mitochondrial Transfer Efficacy in Preclinical AKI Models
Evidence from preclinical studies robustly supports the therapeutic potential of MSC-mediated mitochondrial transfer in AKI. The following table summarizes key quantitative findings from recent research.
Table 2: Efficacy of MSC-Mediated Mitochondrial Transfer in Preclinical AKI Models
| AKI Model / Intervention | Key Measured Outcomes | Reported Results | Proposed Primary Mechanism |
|---|---|---|---|
| Cisplatin-induced AKIMSC co-culture with damaged renal cells | Mitochondrial function restorationReduction in tubular cell apoptosis | Recovery of aerobic respirationSignificant decrease in cell death markers | Mitochondrial donation via TNTs [25] |
| Ischemia-Reperfusion Injury (IRI)Miro1-overexpressing MSCs | Mitochondrial retention in renal tissueFunctional recovery | Enhanced functional kidney recoveryImproved survival of tubular cells | Enhanced TNT-mediated transfer [25] |
| Cisplatin-induced AKIBMSC-derived Exosomes (BMSCs-exo) | Serum creatinine & urea nitrogenTubular injury markers (NGAL, KIM1) | Significant reduction in creatinine/ureaDownregulation of NGAL and KIM1 | EV-mediated paracrine signaling & component transfer [27] |
| General AKI ModelsAdipose-derived MSC-EVs (AMSC-EVs) | Macrophage polarizationInflammatory cytokine levels | Promotion of anti-inflammatory M2 phenotypeAlteration of inflammatory microenvironment | EV-mediated modulation of TXNIP-IKKα/NFκB signaling [12] |
Experimental Protocols for Evaluating Mitochondrial Transfer
Protocol: In Vitro Co-culture System for Visualizing Mitochondrial Transfer
Objective: To directly observe and quantify the transfer of mitochondria from MSCs to cisplatin-injured renal tubular epithelial cells (TECs) in a controlled environment.
Materials:
- Primary Human Renal Proximal Tubular Epithelial Cells (RPTECs): Target recipient cells.
- Human MSCs: Isolated from bone marrow (BMSCs) or adipose tissue (AMSCs). Use passages 3-8.
- Cell Culture Trackers: MitoTracker Deep Red (for MSC mitochondria, red fluorescence) and MitoTracker Green (for total mitochondria, green fluorescence).
- Cisplatin: Nephrotoxic agent to induce injury in TECs.
- Confocal Live-Cell Imaging System: For real-time visualization.
Procedure:
- Cell Preparation and Labeling:
- Culture RPTECs in a 2-chamber co-culture system until 70% confluency.
- Induce injury by adding cisplatin (1-5 µg/mL) for 24 hours.
- Simultaneously, label MSCs with MitoTracker Deep Red (100 nM) for 30 minutes to stain their mitochondria.
- Wash MSCs thoroughly to remove excess dye.
- Co-culture and Imaging:
- Add labeled MSCs to the injured RPTECs in a ratio of 1:10 (MSC:RPTEC).
- After 6-24 hours of co-culture, counterstain all cells with MitoTracker Green (200 nM) for 30 minutes to identify total mitochondrial network.
- Fix cells and image using a confocal microscope with appropriate laser lines.
- Quantification: Mitochondrial transfer is confirmed by the presence of double-positive puncta (red from MSCs within green TECs) and quantified as the percentage of TECs containing MSC-derived mitochondria.
Protocol: In Vivo Evaluation of Mitochondrial Transfer Following Renal Cortex Injection
Objective: To assess the therapeutic efficacy and mitochondrial transfer of MSCs delivered via renal cortex injection in a murine model of cisplatin-induced AKI.
Materials:
- Animals: C57BL/6 mice (8-10 weeks old).
- MSCs: Expressing a fluorescent mitochondrial reporter (e.g., Mito-DsRed).
- Cisplatin: Single intraperitoneal injection (10-15 mg/kg).
- In Vivo Imaging System (IVIS): For tracking cell localization.
- Reagents for Renal Function: Kits for serum creatinine and blood urea nitrogen (BUN).
Procedure:
- AKI Induction and MSC Administration:
- Induce AKI in mice via a single intraperitoneal injection of cisplatin.
- 24 hours post-injury, anesthetize mice and perform a flank incision to expose the kidney.
- Using a Hamilton syringe with a 30-gauge needle, slowly inject 1-2 x 10^5 MSCs suspended in 50 µL of PBS directly into the renal cortex at multiple sites to minimize backflow.
- Include control groups: sham-operated and cisplatin-injected mice receiving PBS only.
- Tissue Collection and Analysis:
- 72-96 hours post-MSC injection, collect blood for creatinine/BUN analysis.
- Perfuse mice with cold PBS, then harvest kidneys.
- Process kidney tissue for:
- Histology (PAS staining): Assess tubular injury score.
- Immunofluorescence: Stain for TEC markers (e.g., Aquaporin-1) and analyze co-localization with the Mito-DsRed signal to confirm mitochondrial transfer in vivo.
- Biochemical Assays: Homogenize cortical tissue to measure ATP levels (using a luciferase-based assay) and ROS (using DCFDA fluorescence).
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for Investigating Mitochondrial Transfer in AKI
| Reagent / Material | Function / Application | Example Use Case |
|---|---|---|
| MitoTracker Probes | Fluorescent dyes that label live-cell mitochondria. | Distinguishing donor vs. recipient mitochondria in co-culture experiments [25]. |
| MSCs with Fluorescent Mitochondrial Reporters | Genetically encoded tags (e.g., Mito-DsRed, Mito-GFP) for stable mitochondrial labeling. | Long-term tracking of transferred mitochondria in vitro and in vivo. |
| Cisplatin | Chemotherapeutic agent used to establish nephrotoxic AKI models. | Inducing mitochondrial damage and dysfunction in renal tubular cells [27]. |
| Miro1-Overexpressing MSCs | Genetically modified MSCs with enhanced mitochondrial motility. | Boosting the efficiency of TNT-mediated mitochondrial transfer in therapeutic applications [25]. |
| Extracellular Vesicle Isolation Kits | Ultracentrifugation or polymer-based kits for purifying MSC-EVs. | Investigating the EV-mediated mitochondrial transfer pathway [12] [27]. |
| Antibodies for CD9, CD63, CD81 | Surface markers for characterizing and validating isolated extracellular vesicles. | Confirming the successful isolation of EVs from MSC conditioned media [12]. |
Mitochondrial transfer represents a paradigm shift in understanding how MSCs facilitate tissue repair in AKI, moving beyond traditional paracrine mechanisms to direct organelle-based bioenergetic rescue. The protocols outlined herein for visualizing and quantifying this process—from in vitro co-cultures to renal cortex injection models—provide a robust framework for researchers to explore and enhance this novel therapeutic avenue. As the field advances, strategies to improve the efficiency of mitochondrial transfer, such as MSC preconditioning or genetic engineering, hold significant promise for developing next-generation regenerative therapies for acute kidney injury.
Cell replenishment is critical for adult tissue repair after damage. Unlike the liver, the kidney has a limited inherent regeneration capacity. For years, it was even considered unable to regenerate itself [29] [30]. However, recent research has demonstrated that mesenchymal stem cells (MSCs) contribute significantly to renal repair primarily through paracrine mechanisms rather than direct differentiation [29] [30] [31]. When MSCs are administered, they secrete a diverse array of bioactive molecules that collectively create a regenerative milieu capable of constraining renal damage and amplifying endogenous repair processes [29] [30].
Among the numerous factors secreted by MSCs, Hepatocyte Growth Factor (HGF), Vascular Endothelial Growth Factor (VEGF), and Insulin-like Growth Factor-1 (IGF-1) have emerged as crucial mediators of tubular regeneration [29] [30] [31]. These factors work in concert to modulate inflammation, inhibit apoptosis, counteract oxidative stress, and stimulate the proliferation of surviving tubular epithelial cells [31] [20]. This Application Note delineates the specific roles, mechanisms, and experimental assessment methodologies for these key paracrine factors within the context of MSC-based therapy for Acute Kidney Injury (AKI), providing researchers with essential protocols for investigating tubular regeneration.
Factor-specific Mechanisms and Assessment
Hepatocyte Growth Factor (HGF)
Mechanisms in Tubular Regeneration: HGF demonstrates potent anti-apoptotic, anti-inflammatory, and mitogenic properties specifically targeted to renal tubules. It inhibits TLR2 and TLR4 signaling pathways, thereby alleviating high glucose-induced inflammatory responses in podocytes and tubular cells [20]. Furthermore, HGF plays a role in reversing epithelial-to-mesenchymal transition (EMT), a key process in fibrosis development, thereby preserving the epithelial phenotype of tubular structures and preventing pathological transition to a profibrotic state [32].
Quantitative Assessment of HGF Efficacy: Table: Experimental Outcomes of HGF-Associated Interventions in Preclinical AKI Models
| Intervention Type | AKI Model | Key Efficacy Parameters | Outcome Measures | Reference |
|---|---|---|---|---|
| hUMSCs secreting HGF | Diabetic Nephropathy Mice | Glomerulosclerosis, Renal Fibrosis, TLR2/4 Pathway | Significant reduction in fibrosis markers and inflammatory pathway activation | [20] |
| MSC-Conditioned Medium (HGF-rich) | Albumin-Induced Tubulinjury | Tubular Inflammation, Fibrosis | Amelioration of tubular injury via HGF and TSG-6 | [31] |
| Low Serum Cultured Adipose Stromal Cells | Acute Kidney Injury | Tubular Repair, Functional Recovery | Primary mediator of renoprotective effect identified as HGF | [31] |
Vascular Endothelial Growth Factor (VEGF)
Mechanisms in Tubular Regeneration: VEGF is a master regulator of angiogenesis, the process of forming new blood vessels. It is critically important for restoring the peritubular capillary network that is often compromised during ischemic AKI [29] [33]. By promoting endothelial cell survival, proliferation, and migration, VEGF ensures adequate oxygen and nutrient delivery to the regenerating tubules, facilitating their repair [11]. Its expression is upregulated in early stages of chronic allograft nephropathy, highlighting its role in the initial tissue response to injury [34].
Quantitative Assessment of VEGF Efficacy: Table: Experimental Outcomes of VEGF-Associated Interventions in Preclinical AKI Models
| Intervention Type | AKI Model | Key Efficacy Parameters | Outcome Measures | Reference |
|---|---|---|---|---|
| Hypoxic Preconditioned MSCs (↑VEGF) | Gentamicin-Induced Renal Failure | Renal Function, HGF/VEGF/Integrin Expression | Ameliorated renal function, increased VEGF expression | [11] |
| 5% O₂ Preconditioned AD-MSCs | Ischemia-Reperfusion Injury | EVs Number, Protein Concentration, Oxidative Reactions | Reduced oxidative stress, protected kidney function | [11] |
| Growth Factor-Modified MSCs | Acute Kidney Injury | Renal Repair, Capillary Density | Superior therapeutic potential via paracrine signaling | [33] |
Insulin-like Growth Factor-1 (IGF-1)
Mechanisms in Tubular Regeneration: IGF-1 is a powerful stimulator of cellular proliferation and differentiation. In the context of tubular repair, it enhances the proliferation and dedifferentiation of surviving tubular epithelial cells, enabling them to repopulate damaged segments of the nephron [29] [33]. IGF-1 signaling activates downstream pathways such as PI3K/Akt and MAPK, which promote cell cycle progression and inhibit pro-apoptotic signals, thus creating a favorable environment for tubular regeneration [31]. Its signaling pathway is significantly upregulated in moderate to severe chronic kidney injury, indicating a sustained role in the repair process [34].
Quantitative Assessment of IGF-1 Efficacy: Table: Experimental Outcomes of IGF-1-Associated Interventions in Preclinical AKI Models
| Intervention Type | AKI Model | Key Efficacy Parameters | Outcome Measures | Reference |
|---|---|---|---|---|
| Human Amniotic Fluid Stem Cells | Cisplatin-Induced Kidney Injury | Paracrine Mediators (IGF-1, IL-6, VEGF) | Renoprotective effect mediated by IGF-1 and other factors | [31] |
| Bone Marrow-Derived MSCs | Ischemic Acute Kidney Injury | Apoptosis Inhibition, Endogenous Cell Proliferation | Protected kidney via trophic factors including IGF-1 | [31] |
| Meta-analysis of CAN/IFTA | Transplant Nephropathy | IGF-1 Signaling Pathway | Significant upregulation in moderate/severe chronic injury | [34] |
Integrated Signaling Pathways in Tubular Regeneration
The factors HGF, VEGF, and IGF-1 mediate their regenerative effects through interconnected signaling networks that coordinate the repair of damaged renal tubules. The following diagram illustrates the key pathways and their cellular outcomes:
Experimental Protocols for Factor Analysis
Protocol: In Vivo Assessment of Paracrine Factors in MSC-Treated AKI
Objective: To evaluate the therapeutic efficacy and mechanism of action of MSC-derived paracrine factors (HGF, VEGF, IGF-1) in a rodent model of gentamicin-induced AKI [3].
Materials:
- Sprague-Dawley rats (200-250g)
- Gentamicin sulfate
- MSC population (e.g., Bone Marrow-MSCs, Tonsil-MSCs, or Adipose Tissue-MSCs)
- PKH26 fluorescent cell linker dye
- ELISA kits for HGF, VEGF, IGF-1, BUN, Creatinine
- Antibodies for TUNEL staining, Bax, Bcl-2, KIM-1, NGAL
Procedure:
- AKI Induction: Administer gentamicin (70 mg/kg/day, i.p.) for 10 consecutive days.
- MSC Preparation and Labeling:
- Culture MSCs to 80% confluence in standard conditions.
- Label 1×10^7 cells with PKH26 dye according to manufacturer's protocol for in vivo tracking.
- Cell Administration: On day 11, inject labeled MSCs via tail vein in 500μL saline solution.
- Tissue and Fluid Collection: On day 16, sacrifice animals and collect:
- Blood for BUN and creatinine measurement
- Urine for biomarker analysis (KIM-1, NGAL, 8-OHdG)
- Kidney tissue for histology and protein analysis
- Tract MSCs: Localize PKH26-labeled MSCs in renal cortex using fluorescence microscopy.
- Assess Renal Function: Quantify BUN and creatinine levels using commercial assay kits.
- Evaluate Apoptosis: Perform TUNEL staining on kidney sections; analyze apoptotic ratio (TUNEL-positive cells/total cells).
- Measure Oxidative Stress: Quantify urinary 8-OHdG using oxidative DNA damage ELISA kit.
- Analyze Factor Expression: Determine HGF, VEGF, and IGF-1 levels in serum and renal tissue lysates via ELISA.
Expected Outcomes: The GM+MSC group should show significantly lower BUN, creatinine, tubular damage scores, apoptosis, and oxidative stress markers compared to the GM-only group. PKH26 staining should demonstrate MSC localization in renal tubules, confirming engraftment.
Protocol: In Vitro Coculture System for Paracrine Factor Analysis
Objective: To investigate the direct paracrine effects of MSCs on injured renal tubular epithelial cells (NRK cells) in a controlled microenvironment [3].
Materials:
- NRK-52E rat renal tubular epithelial cell line
- MSC population (any source)
- Transwell coculture system (0.4μm pore size)
- Gentamicin for injury induction
- Conditioned media collection facilities
- HGF, VEGF, IGF-1 neutralizing antibodies
- Apoptosis detection kit (Annexin V/PI)
- DCFDA assay for ROS detection
Procedure:
- Cell Culture Setup:
- Plate NRK-52E cells in the lower chamber of transwell system.
- Plate MSCs in the upper chamber insert.
- Tubular Cell Injury: Add gentamicin (2-5mM) to both chambers for 24-48 hours.
- Conditioned Media Collection: Harvest media from coculture system after 48 hours for subsequent factor analysis.
- Viability and Apoptosis Assessment:
- Detect apoptosis in NRK cells using Annexin V/PI flow cytometry.
- Measure cell viability via MTT assay.
- Oxidative Stress Measurement: Quantify intracellular ROS in NRK cells using DCFDA fluorescent dye.
- Factor Neutralization Studies:
- Add neutralizing antibodies against HGF, VEGF, or IGF-1 individually or in combination to the coculture system.
- Assess which antibody treatment most significantly abrogates the protective effects of MSCs.
- Pathway Analysis: Perform Western blotting on NRK cell lysates to analyze activation of PI3K/Akt, MAPK, and other downstream pathways.
Expected Outcomes: MSC coculture should significantly reduce gentamicin-induced apoptosis and ROS generation in NRK cells. Neutralization of HGF, VEGF, and IGF-1 should partially reverse these protective effects, confirming their role in the paracrine mechanism.
The Scientist's Toolkit: Essential Research Reagents
Table: Key Reagents for Investigating Paracrine Factors in Renal Regeneration
| Reagent / Assay | Specific Example | Research Application | Experimental Function |
|---|---|---|---|
| ELISA Kits | HGF, VEGF, IGF-1 ELISA | Quantification in serum, urine, tissue lysates | Measures concentration of target growth factors |
| Neutralizing Antibodies | Anti-HGF, Anti-VEGF, Anti-IGF-1 | In vitro coculture neutralization studies | Blocks specific factor activity to confirm mechanistic role |
| Cell Tracking Dyes | PKH26, PKH67 | In vivo cell fate studies | Fluorescently labels MSCs for localization post-transplantation |
| Renal Injury Markers | KIM-1, NGAL ELISA | Assessment of tubular damage | Quantifies severity of kidney injury and recovery |
| Apoptosis Assays | TUNEL Staining, Annexin V/PI | Detection of programmed cell death | Evaluates anti-apoptotic effects of paracrine factors |
| Oxidative Stress Kits | 8-OHdG ELISA, DCFDA Assay | Measurement of ROS and DNA damage | Assesses oxidative stress levels in renal tissue and cells |
| Pathway Inhibitors | PI3K/Akt inhibitors, MAPK inhibitors | Mechanistic signaling studies | Blocks specific downstream pathways to elucidate mechanisms |
Strategic Enhancement of MSC Paracrine Activity
Research indicates that the native paracrine activity of MSCs can be substantially enhanced through various preconditioning strategies to improve therapeutic outcomes for AKI [11]:
Hypoxic Preconditioning: Culturing MSCs under low oxygen conditions (1-5% O₂) significantly enhances their secretion of HGF, VEGF, and other regenerative factors, improving their survival, migration, and therapeutic potential after transplantation into the ischemic kidney microenvironment [11].
Pharmacological Preconditioning: Treatment with FDA-approved drugs like Chlorzoxazone can induce MSCs to adopt an anti-inflammatory phenotype, strengthening their immunosuppressive capacity without increasing immunogenicity. Similarly, Atorvastatin pretreatment synergistically enhances the inherent therapeutic effects of BMSCs [11].
Genetic Modification: Engineering MSCs to overexpress specific growth factors (HGF, VEGF) or pro-survival genes further amplifies their paracrine activity and renal protective effects, offering a promising strategy to maximize therapeutic efficacy [11] [33].
The paracrine factors HGF, VEGF, and IGF-1 serve as critical mediators of tubular regeneration in MSC-based therapies for AKI. Through their coordinated actions on multiple cellular processes—including apoptosis inhibition, proliferation stimulation, angiogenesis, and inflammation modulation—these factors create a regenerative microenvironment conducive to renal repair. The protocols and methodologies outlined in this Application Note provide researchers with robust tools for investigating these mechanisms and developing enhanced therapeutic strategies for acute kidney injury.
Technical Implementation of Renal Cortex Injection for MSC Delivery
A critical challenge in mesenchymal stem cell (MSC)-based therapy for acute kidney injury (AKI) is the efficient delivery of cells to the target site. The administration route directly determines the initial biodistribution of MSCs, influencing engraftment, retention, and ultimate therapeutic efficacy [35] [36]. This application note examines the fundamental advantage of local administration strategies, particularly renal cortex injection, over systemic delivery, with a focus on the critical mechanism of bypassing pulmonary trapping to enhance renal-specific homing. This is framed within the broader research context of optimizing MSC paracrine therapy for AKI, where delivering a sufficient concentration of cells to the injured renal tissue is paramount for harnessing their reparative effects.
Comparative Analysis: Local vs. Systemic Administration
The Pulmonary First-Pass Effect in Systemic Administration
Intravenous (IV) infusion, the most common systemic route, is hindered by the pulmonary first-pass effect. Upon injection, a significant proportion of MSCs are physically trapped in the capillary networks of the lungs [35] [36]. This occurs because MSCs are larger than most capillaries and exhibit adhesive interactions with the pulmonary endothelium [36]. The liver also sequesters a considerable number of cells [35]. This nonspecific distribution results in an inadequate therapeutic concentration of MSCs reaching the kidneys and necessitates higher, potentially risky, cell doses to achieve a therapeutic effect in the target organ [35] [36].
Local Administration for Targeted Delivery
In contrast, local administration methods, such as direct renal injection, inoculate MSCs precisely into the kidney, circumventing the systemic circulation and the pulmonary filter [35] [2]. This approach significantly increases the number of cells initially present at the site of injury, enhancing the potential for local engraftment and paracrine activity [35].
Table 1: Quantitative Comparison of Administration Routes for MSC Delivery to Kidney
| Administration Route | Key Advantage | Primary Limitation | Reported Renal Retention/Outcome |
|---|---|---|---|
| Intravenous (IV) | Minimally invasive, simple [35] | High pulmonary/liver sequestration; low renal delivery [35] [36] | Low engraftment; inadequate therapeutic concentration [35] |
| Intra-arterial (IA) | Bypasses pulmonary filter [35] | Risk of cell embolism; more traumatic [35] | More efficacious than IV, but safety concerns [35] |
| Local (e.g., Renal Cortex/Parenchymal Injection) | Direct delivery to site of injury; avoids pulmonary trap [35] [2] | More invasive; potential for injection-site injury [35] | High initial retention; superior functional and histological improvement in preclinical AKI models [35] [37] |
Table 2: Preclinical Evidence Supporting Local Administration for AKI
| Reference | Year | Animal Model | MSC Source | Local Injection Method | Key Renal Outcomes |
|---|---|---|---|---|---|
| Huang et al. [35] | 2022 | Mice (I/R) | Umbilical Cord | Subcapsular / Parenchymal | Improved renal function and tubular repair; Reduced injury and fibrosis |
| Wang et al. [35] | 2020 | Mice (I/R) | Human Placenta | Subcortical | Recovery of function; Facilitated angiogenesis; Decreased fibrosis |
| Paglione et al. [35] | 2020 | Rats (I/R) | Human Omental | Parenchymal | Accelerated functional recovery; Ameliorated tubular injury |
| Frontiers in Cell and Developmental Biology [37] | 2024 | Mice (UUO) | Human Adipose | Local vs. Systemic | Local delivery reduced collagen deposition and increased IL-10; Systemic administration showed no significant effect. |
Experimental Protocols for Local Renal Administration
Protocol 1: Ultrasound-Guided Renal Artery Injection in Mice
This protocol describes a minimally invasive method for local delivery of MSCs to the kidney via the renal artery, adapted from a study that achieved high initial renal cell retention [38].
Key Materials:
- Luciferase/GFP-expressing human Adipose-Derived Stem Cells (ADSCs)
- Silica-coated Gold Nanorods (GNRs) for photoacoustic imaging
- 0.6% (w/v) Alginate solution in saline
- Small animal ultrasound system with high-frequency transducer (e.g., 40 MHz)
- Isoflurane anesthesia system
Methodology:
- Cell Preparation: Label ADSCs with silica-coated GNRs (incubate for 24 hours). Post-incubation, wash and resuspend 2x10^5 cells in 100 µL of 0.6% alginate solution [38].
- Animal Preparation: Anesthetize a 6-8 week old female nude mouse and position it in the left lateral decubitus position. Maintain body temperature at 37°C [38].
- Ultrasound Guidance: Using the ultrasound platform, acquire a colour Doppler image of the right kidney to visualize the renal artery and vein. Use pulsed-wave Doppler to confirm vessel identity based on flow waveforms [38].
- Injection Procedure: Under real-time ultrasound guidance, advance a needle through the paravertebral muscle and into the renal artery. Slowly inject the 100 µL cell-alginate suspension over approximately 15 seconds. Hyperechogenicity from the alginate will be visible around the renal cortex upon successful injection [38].
- Post-injection: Hold the needle in place for 15 seconds after injection before slow withdrawal to prevent backflow. Confirm return of renal artery flow via colour Doppler within 20 seconds post-injection [38].
- Cell Tracking: Serially track cell viability and localization using bioluminescence imaging (BLI) and photoacoustic (PA) imaging over 7 days. This study reported approximately 29% of the total BLI signal in the target kidney at 1 hour post-injection, with signals persisting for 3 days [38].
Protocol 2: Direct Renal Parenchymal/Cortex Injection
This method involves the direct injection of MSCs into the renal tissue, a common approach in preclinical AKI studies [35].
Key Materials:
- Bone Marrow or Adipose-derived MSCs
- Phosphate Buffered Saline (PBS) or Hydrogel carrier (e.g., Alginate, Collagen)
- Sterile surgical instruments for laparotomy
- Hamilton syringe with a 30-gauge needle
Methodology:
- Cell Preparation: Harvest and resuspend MSCs (e.g., 1x10^6 cells) in an appropriate carrier, such as 50 µL of PBS or a biocompatible hydrogel, to enhance retention [35] [2].
- Surgical Exposure: Perform a minimally invasive laparotomy on an anesthetized rodent (e.g., rat or mouse) to expose the kidney.
- Injection Procedure: Using a Hamilton syringe, carefully inject the cell suspension at multiple sites (e.g., 2-3 sites) into the renal cortex or subcapsular space to distribute the cells. Control injection speed to minimize tissue damage and backflow.
- Post-injection Monitoring: After injection, return the kidney to the abdominal cavity and close the surgical site. Monitor animals for recovery and signs of distress. Assess renal function (serum creatinine, BUN) and histology (H&E, Masson's Trichrome) at predetermined endpoints to evaluate efficacy [35].
Visualization of Administration Routes and Cell Fate
MSC Administration Routes and Biodistribution
Experimental Workflow for Local MSC Delivery
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Local MSC Delivery Experiments
| Research Reagent / Material | Function / Application | Example Use Case |
|---|---|---|
| Gold Nanorods (GNRs), silica-coated | High-contrast agent for photoacoustic cell tracking; allows non-invasive visualization of MSC localization post-delivery [38]. | Tracking ADSCs in mouse kidney for 7 days post ultrasound-guided renal artery injection [38]. |
| Alginate Solution (0.6% w/v) | Biocompatible carrier polymer; improves cell retention during injection and provides real-time ultrasound contrast [38]. | Used as vehicle for suspending MSCs in renal artery injection protocol [38]. |
| Luciferase/GFP-expressing MSCs | Genetic reporter system enabling bioluminescence imaging (BLI) for cell viability and quantitative tracking [38]. | Monitoring viability and persistence of ADSCs in mouse kidney over time [38]. |
| Hydrogels (e.g., Collagen, Hyaluronic Acid) | Natural or synthetic polymer scaffolds for 3D cell culture and delivery; act as artificial extracellular matrix to enhance MSC survival and paracrine function [35] [2]. | Used in direct parenchymal injection to create a protective microenvironment for MSCs [35]. |
| Preconditioning Agents (e.g., TNF-α, IFN-γ) | Cytokines used to pretreat MSCs, enhancing their immunomodulatory and anti-fibrotic paracrine profile prior to transplantation [11] [37]. | Boosting therapeutic efficacy of adipose-derived MSCs in a murine model of chronic kidney disease [37]. |
Precision injection into the renal parenchyma represents an advanced delivery strategy for mesenchymal stem cell (MSC)-based therapies for Acute Kidney Injury (AKI). This technique aims to overcome significant limitations of systemic administration routes by enabling direct, localized delivery of therapeutic cells to the injured kidney. Systemic intravenous (IV) infusion, while less invasive, results in substantial cell trapping in extracranial organs—primarily the lungs and liver—leading to low renal retention rates that often fall below 1-2% of administered cells [11] [39]. In contrast, direct parenchymal injection facilitates maximal local cell density at the injury site, potentially enhancing paracrine-mediated repair processes that underlie MSC efficacy in AKI.
The therapeutic benefits of MSCs in AKI are now understood to be mediated predominantly through complex paracrine actions rather than direct engraftment and differentiation [10] [40] [1]. MSCs secrete a diverse array of bioactive molecules—including cytokines, chemokines, growth factors, and extracellular vesicles—that collectively promote tissue repair through multiple mechanisms: immunomodulation, anti-apoptosis, anti-oxidation, and stimulation of angiogenesis [11] [41] [42]. Precision parenchymal injection ensures these paracrine factors are delivered directly to the compromised renal tissue, creating a localized therapeutic microenvironment that can accelerate recovery and potentially prevent progression to chronic kidney disease.
Quantitative Analysis of MSC Delivery Strategies
Research comparing different administration routes provides critical insights for optimizing MSC delivery. The following table summarizes key findings from preclinical and clinical studies evaluating various injection methodologies for MSC therapy in kidney disease:
Table 1: Comparison of MSC Delivery Routes for Kidney Therapy
| Delivery Route | Reported Cell Retention | Therapeutic Efficacy (SUCRA Value) | Key Advantages | Major Limitations |
|---|---|---|---|---|
| Intravenous (IV) | <1-2% in kidney [39] | 55.2% [39] | Minimally invasive, widespread distribution | High pulmonary/liver sequestration, low target organ retention |
| Intra-arterial (IA) | ~4% in healthy kidneys, ~12% in stenotic kidneys [10] | 44.2% [39] | Higher renal retention than IV, first-pass renal effect | Potential embolization risk, requires specialized catheterization skills |
| Intrarenal/Parenchymal | Not quantitatively reported in literature; presumed highest local concentration | 67.9% [39] | Maximum local cell density, minimal systemic loss | Technically challenging, potential for local tissue injury, invasive procedure |
Table 2: MSC Dosing Strategies in AKI Models
| Dose Category | Cell Quantity | Therapeutic Efficacy (SUCRA Value) | Clinical Context |
|---|---|---|---|
| Low Dose | ≤1×10⁶ cells [39] | 31.9% [39] | Common starting point for initial clinical trials |
| High Dose | >1×10⁶ cells [39] | 68.1% [39] | Associated with superior functional recovery in meta-analyses |
Network meta-analysis of transplantation strategies has demonstrated that direct intrarenal delivery achieves the highest surface under the cumulative ranking curve (SUCRA) value (67.9%), followed by intravenous (55.2%) and intra-arterial (44.2%) routes, though statistical significance between routes was not always achieved [39]. Similarly, high-dose regimens (>1×10⁶ cells) significantly outperform lower doses (SUCRA 68.1% vs. 31.9%) in preclinical models [39].
Experimental Protocol: Precision Renal Parenchyma Injection
Preoperative Preparation
MSC Source and Characterization: Isplicate MSCs from bone marrow (BM-MSC), adipose tissue (AD-MSC), or umbilical cord (UC-MSC) following established protocols [41] [42]. Validate cells according to International Society for Cellular Therapy (ISCT) criteria: confirm plastic adherence, expression of CD73, CD90, CD105 (>95%), and absence of CD14, CD34, CD45, HLA-DR (<2%) [41]. Assess multipotent differentiation potential (osteogenic, adipogenic, chondrogenic) and exclude senescent cells using β-galactosidase staining [41].
Cell Processing and Labeling: Harvest MSCs at 80-90% confluence (passage 3-5). For tracking purposes, label cells with fluorescent markers (e.g., DiI, GFP) or superparamagnetic iron oxide nanoparticles for MRI detection [10]. Resuspend in sterile saline at a concentration of 50,000-100,000 cells/μL for injection, maintaining viability >95% by trypan blue exclusion.
Animal Preparation and Anesthesia: Utilize adult rodent AKI models (e.g., ischemia-reperfusion injury, cisplatin-induced nephrotoxicity). Induce anesthesia with ketamine/xylazine (rodents) or isoflurane (larger species). Secure animal in right lateral decubitus position for left kidney exposure. Maintain body temperature at 37°C throughout procedure using heating pad.
Surgical Procedure
Surgical Exposure: Perform aseptic preparation of surgical site. Make a 1.5-2cm dorsal incision parallel and 1cm lateral to the spine. Carefully dissect through muscle layers to expose the peritoneal membrane. Identify the kidney through the membrane and gently exteriorize it using moistened cotton swabs. Place moistened gauze around the organ to prevent desiccation.
Injection Technique: Utilize a micro-syringe pump system with a 30-gauge needle for precise control. Insert needle at a 30-45° angle through the renal capsule into the cortical parenchyma, avoiding the medulla and hilum. Administer cells slowly (50-100μL/min) to minimize reflux and tissue damage. Multiple injections (typically 3-5) may be performed throughout the cortex, spaced at least 2mm apart. Limit volume to 10-20μL per injection site in rodent models to prevent compartment syndrome.
Closure and Recovery: After injection, apply gentle pressure with sterile gelatin sponge or surgical foam to achieve hemostasis. Carefully return the kidney to the retroperitoneal space. Close muscle layers with absorbable sutures (e.g., 4-0 Vicryl) and skin with surgical staples or non-absorbable sutures. Administer postoperative analgesia (buprenorphine) and monitor animals until fully recovered from anesthesia.
Post-procedure Assessment
Functional Monitoring: Track renal function parameters at 24, 48, and 72 hours post-procedure: serum creatinine (SCr), blood urea nitrogen (BUN), glomerular filtration rate (GFR), and urine output [39].
Histological Analysis: At endpoint, process kidney tissue for histological assessment: hematoxylin and eosin staining for tubular injury scoring, Periodic acid-Schiff staining for brush border integrity, Masson's trichrome for fibrosis evaluation, and immunofluorescence for tracking labeled cells and assessing engraftment.
Molecular Analysis: Analyze tissue for paracrine factor expression (VEGF, HGF, IGF-1) [10], inflammatory markers (TNF-α, IL-6, IL-10) [11], and apoptosis (TUNEL assay, caspase-3 activation) to elucidate mechanisms of repair.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for Renal Parenchyma Injection Studies
| Reagent/Category | Specific Examples | Research Function |
|---|---|---|
| MSC Isolation Kits | Human BM-MACs, Adipose Stem Cell Isolation Kit | Isolation and purification of MSCs from tissue sources |
| Cell Culture Media | MesenCult, StemMACS MSC Expansion Media | In vitro expansion while maintaining multipotency |
| Cell Labeling Agents | DiI, CM-Dil, GFP-lentivirus, SPIO nanoparticles | Cell tracking post-transplantation |
| AKI Model Inducers | Cisplatin, glycerol, ischemia-reperfusion surgery | Creation of controlled kidney injury models |
| Analytical Antibodies | Anti-CD73/90/105, anti-CD34/45/14, anti-HLA-DR | MSC characterization via flow cytometry |
| Histology Reagents | H&E, PAS, Masson's Trichrome, TUNEL assay kits | Assessment of renal pathology and repair |
| Renal Function Assays | Creatinine assay kit, BUN test kits | Quantification of functional recovery |
Signaling Pathways in MSC-Mediated Renal Repair
The therapeutic benefits of precision-injected MSCs are mediated through complex paracrine signaling mechanisms that modulate multiple repair pathways simultaneously. The following diagram illustrates key signaling pathways activated by MSC therapy in AKI:
MSC Signaling Pathways in AKI Recovery
Technical Considerations and Optimization Strategies
Enhancing MSC Efficacy Through Preconditioning
Maximizing the therapeutic potential of injected MSCs often requires preconditioning strategies to enhance their survival and paracrine activity in the hostile inflammatory environment of injured kidney tissue:
Hypoxic Preconditioning: Culture MSCs at 1-5% O₂ for 24-48 hours prior to injection. This upregulates HIF-1α and CXCR4 expression, enhancing cell survival, migration, and secretion of pro-angiogenic factors (VEGF, HGF) [11]. Studies demonstrate hypoxia-preconditioned MSCs exhibit improved anti-oxidative capacity and renal functional recovery in IRI models [11].
Pharmacological Preconditioning: Pretreat MSCs with chlorzoxazone (FDA-approved muscle relaxant) to enhance anti-inflammatory phenotype through FoxO3 phosphorylation [11]. Alternatively, atorvastatin preconditioning augments anti-apoptotic, antioxidant, and anti-inflammatory properties of MSCs [11].
3D Culture Systems: Utilize spheroid culture or hydrogel encapsulation to better mimic the native MSC microenvironment. These approaches enhance cell-cell interactions and paracrine factor secretion compared to conventional 2D culture [11].
Injection Parameter Optimization
Successful implementation requires careful optimization of technical parameters:
Needle Gauge Selection: Balance between minimizing tissue trauma (smaller gauge: 31-33G) and preventing cell shear stress (larger gauge: 27-30G). 30-gauge needles typically offer optimal compromise for rodent models.
Injection Volume and Rate: Strictly control injection parameters based on species: rodents (10-20μL at 50-100nL/sec), swine (100-200μL at 200-500nL/sec), non-human primates (200-500μL at 500nL-1μL/sec). Use microprocessor-controlled injection systems for reproducibility.
Spatial Distribution: Target multiple cortical sites while avoiding medullary regions and major vessels. Computational modeling suggests hexagonal injection patterns with 2-3mm spacing optimize distribution in rodent kidneys.
Precision injection into the renal parenchyma represents a sophisticated delivery platform for MSC-based therapies in AKI, offering distinct advantages over systemic administration routes through enhanced local cell retention and targeted paracrine signaling. When integrated with MSC preconditioning strategies and optimized injection parameters, this approach maximizes the therapeutic potential of MSCs to modulate complex injury responses and promote renal repair. As the field advances, continued refinement of this technique—potentially combined with innovative biomaterial scaffolds and targeted genetic modifications—will further enhance its translational potential for treating acute kidney injury and preventing its progression to chronic kidney disease.
The therapeutic efficacy of Mesenchymal Stem Cells (MSCs) in treating Acute Kidney Injury (AKI) is demonstrated through key functional and histological parameters. The following tables summarize quantitative improvements observed in animal models.
Table 1: Renal Function and Tubular Damage in Gentamicin-Induced AKI (Rat Model) [3]
| Parameter | GM Group | GM + T-MSCs Group | Measurement Method |
|---|---|---|---|
| Blood Urea Nitrogen (BUN) | Increased | Significantly Lower | QuantiChrom Urea Nitrogen Assay Kit |
| Serum Creatinine | Increased | Significantly Lower | QuantiChrom Creatinine Assay Kit |
| Tubular Damage Score | Higher (2-4 on 0-4 scale) | Significantly Lower | Periodic acid-Schiff staining; semi-quantitative scoring (0=none, 4=>75% involvement) |
Table 2: Apoptotic and Oxidative Stress Markers in Gentamicin-Induced AKI [3]
| Marker Category | Specific Marker | Change in GM + T-MSCs Group (vs. GM Group) | Assessment Method |
|---|---|---|---|
| Apoptosis-related | Bax / Cytochrome c / Cleaved Caspase | Decreased | Western Blot |
| Bcl-2 | Increased | Western Blot | |
| TUNEL-positive cells | Decreased | TdT-mediated dUTP Nick-End Labeling assay | |
| Oxidative Stress | Urinary 8-OHdG | Decreased | Oxidative DNA Damage ELISA Kit |
| Glutathione Peroxidase (GPx) / Catalase | Increased (in kidney tissue) | Western Blot |
Table 3: Efficacy of MSC Preconditioning Strategies in Preclinical AKI Models [11]
| Preconditioning Strategy | AKI Model | Key Improved Outcomes & Proposed Mechanisms |
|---|---|---|
| Hypoxia (1% O₂) | Rat IRI | Improved renal function, reduced apoptosis, enhanced anti-oxidative capacity, increased vascularization, immunomodulation [11]. |
| Hypoxia (5% O₂) | Rat Gentamicin-induced | Increased expression of HGF, VEGF, integrins, and SDF-1, ameliorating renal function [11]. |
| Chemical (Chlorzoxazone) | Mouse Thy1.1 Antibody-induced | Enhanced anti-inflammatory cytokine expression, strengthened immunosuppressive function, reduced renal inflammation and glomerular fibrinoid necrosis [11]. |
Detailed Experimental Protocols
In Vivo Protocol: Renal Cortex Injection of MSCs in Gentamicin-Induced AKI Rats
This protocol details the steps for establishing a gentamicin-induced AKI model in rats and administering MSCs via renal cortex injection to assess functional and histological improvements [3].
Materials Required:
- Animals: Male Sprague-Dawley rats (200-250 g) [3].
- AKI Inducing Agent: Gentamicin (GM) [3].
- Cells: Tonsil-derived MSCs (T-MSCs) or other MSC types [3].
- Labeling Dye: PKH26 fluorescent dye (for cell tracking) [3].
Procedure:
- AKI Induction: Administer gentamicin via daily intraperitoneal injections (70 mg/kg/day) for 10 consecutive days [3].
- Cell Preparation and Labeling:
- MSC Administration (Renal Cortex Injection):
- On day 11 after the first GM injection, perform renal cortex injection [3] [2].
- Anesthetize the rat and surgically expose the kidney.
- Using a micro-syringe (e.g., 30-gauge), slowly inject the cell suspension (e.g., 50-100 μL containing 1-2x10^6 cells) at multiple sites across the renal cortex to maximize distribution and minimize tissue damage.
- Control groups receive an equivalent volume of vehicle solution (saline) [3].
- Termination and Sample Collection:
- On day 16 after the first GM injection, sacrifice the animals [3].
- Collect blood samples for renal function analysis (BUN and creatinine).
- Harvest kidney tissue for histological analysis (fixed in Methyl Carnoy's solution or 4% paraformaldehyde) and protein analysis (snap-frozen in liquid nitrogen).
In Vitro Protocol: Co-culture of MSCs with GM-Injured Renal Tubular Cells
This protocol assesses the direct paracrine effects of MSCs on apoptosis and oxidative stress in injured renal cells [3].
Materials Required:
- Cells: NRK (Normal Rat Kidney) cell line or primary renal tubular epithelial cells; MSCs.
- Culture Vessels: Transwell co-culture system (porous membrane inserts).
- Injury Agent: Gentamicin [3].
- Assessment Kits: Apoptosis detection kit (e.g., TUNEL), Hydrogen peroxide (H₂O₂) detection probe.
Procedure:
- Cell Seeding and Injury:
- Seed NRK cells in the bottom chamber of the culture plate.
- Induce injury by treating NRK cells with a predetermined concentration of gentamicin (e.g., 1-5 mg/mL) for 24 hours.
- Co-culture Establishment:
- Seed MSCs in the upper chamber of the Transwell insert.
- Place the insert into the plate containing the injured NRK cells. This allows for the exchange of soluble paracrine factors without direct cell contact.
- Alternatively, to test only the secreted factors, use T-MSC-conditioned media collected from MSC cultures on the injured NRK cells [3].
- Incubation and Analysis:
Signaling Pathways and Experimental Workflows
The Scientist's Toolkit: Research Reagent Solutions
Table 4: Essential Reagents and Kits for MSC-based AKI Research
| Item Name | Function/Application | Example/Brief Specification |
|---|---|---|
| Gentamicin | Induction of nephrotoxic AKI in animal models. | Administered intraperitoneally at 70 mg/kg/day for 10 days [3]. |
| PKH26 Dye | Fluorescent cell labeling for in vivo cell tracking and localization. | Red fluorescent cell linker; used to trace MSCs to tubules post-injection [3]. |
| QuantiChrom Assay Kits | Accurate measurement of renal function biomarkers. | Urea Nitrogen (BUN) and Creatinine assay kits for serum/urine analysis [3]. |
| TUNEL Assay Kit | Detection of apoptotic cells in tissue sections. | In-situ cell death detection kit (e.g., from Roche) for histological analysis [3]. |
| 8-OHdG ELISA Kit | Quantitative measurement of oxidative DNA damage. | Oxidative DNA Damage ELISA Kit for urine analysis [3]. |
| Transwell Co-culture System | Study of MSC paracrine effects in vitro. | Porous membrane inserts to co-culture MSCs with injured renal cells [3]. |
| Primary Antibodies | Detection of specific proteins via Western Blot/IHC. | Targets: Bax, Bcl-2, Cytochrome c, Cleaved Caspase, KIM-1, NGAL, GPx, Catalase [3]. |
Mesenchymal stem cell (MSC)-based therapy represents a promising regenerative strategy for acute kidney injury (AKI), primarily functioning through paracrine release of bioactive factors that promote tissue repair, modulate inflammation, and inhibit apoptosis [43] [2]. Despite encouraging preclinical results, clinical translation has been hampered by significant biological challenges. A critical limitation lies in the poor retention and survival rates of systemically administered MSCs in target renal tissues, with only a small proportion of transplanted cells successfully homing to injury sites [43] [11]. This technical application note details optimized methodologies for tracking MSC retention following renal cortex injection, providing a standardized framework for evaluating strategies to enhance engraftment for AKI paracrine therapy research.
Quantitative Evidence: MSC Retention and Renal Outcomes
Comprehensive analysis of preclinical and clinical studies reveals a direct correlation between MSC retention and functional renal improvement, while also highlighting the translational gap between animal models and human applications.
Table 1: MSC Therapy Outcomes in Preclinical vs. Clinical Studies
| Parameter | Preclinical (Animal) Models | Clinical (Human) Trials |
|---|---|---|
| TNF-α Reduction | MD = -35.53; 95% CI: -52.75, -18.30; p < 0.0001 [44] | MD = -0.74; 95% CI: -2.20, 0.73; p = 0.32 [44] |
| Serum Creatinine | MD = -0.38; 95% CI: -0.46, -0.29; p < 0.00001 [44] | MD = -0.59; 95% CI: -1.92, 0.74; p = 0.39 [44] |
| Blood Urea Nitrogen | MD = -19.27; 95% CI: -23.50, -15.04; p < 0.00001 [44] | Not Significant [44] |
| Glomerular Filtration Rate | SMD = 1.83; 95% CI: 0.51, 3.15; p = 0.007 [44] | Not Significant [44] |
| Typical MSC Retention | ~12% in stenotic kidneys [10] | Low (exact quantification limited) [43] |
Tracking studies in porcine models of chronic ischemic kidney disease demonstrate that intra-arterially delivered MSCs achieve approximately 12% retention in stenotic kidneys, compared to only 4% in healthy renal tissue, confirming enhanced homing to diseased organs [10]. These retained cells predominantly localize to the renal interstitium and contribute to functional improvement, including significantly improved glomerular filtration rate and renal blood flow [10]. The disparity between strong preclinical results and modest clinical outcomes underscores the necessity of developing more reliable tracking methodologies and retention enhancement strategies for human applications.
Experimental Protocols for MSC Tracking and Retention Analysis
Protocol: Renal Cortex Injection for Targeted MSC Delivery
Principle: Direct intraparenchymal injection bypasses the pulmonary filter and systemic circulation, maximizing local cell delivery while minimizing distribution to non-target organs [2].
Materials:
- MSCs (allogeneic or xenogeneic) labeled with fluorescent marker (e.g., DiI, GFP)
- Small animal stereotactic instrument
- 30-gauge insulin syringe with precision plunger
- Animal warming plate
- Surgical microscope
Procedure:
- Anesthetize and position mouse/rat in lateral decubitus position.
- Perform minimal flank incision to expose the kidney.
- Load 20-50 μL of MSC suspension (5,000-20,000 cells/μL) into injection syringe.
- Under microscopic guidance, slowly inject cell suspension at 2-3 cortical sites along the longitudinal axis.
- Maintain needle position for 60 seconds post-injection to prevent reflux.
- Apply gentle pressure with sterile gel foam at injection site after withdrawal.
- Monitor animals for recovery and provide analgesic care.
Technical Notes: Renal cortex injection allows precise cellular deposition but creates minor, reversible injury at the injection site [2]. This method is particularly valuable for evaluating initial engraftment efficiency before progressing to more complex intravascular delivery models.
Protocol: Longitudinal MSC Tracking via Light Sheet Fluorescence Microscopy
Principle: This advanced imaging technique enables three-dimensional, real-time tracking of fluorescently labeled MSCs within intact renal organoids or tissue with minimal phototoxicity, allowing observation of cell migration and integration over extended periods [45].
Materials:
- Zeiss Z.1 Lightsheet microscope or equivalent system
- Custom PDMS-polycarbonate membrane well constructs
- Fluorophore-labeled MSCs (e.g., CellTracker CM-DiI, GFP-transduced)
- Immersed culture medium (MEM + 10% FCS, without phenol red)
- Hydrogel embedding matrix
Procedure:
- Embed MSC-treated renal organoids or tissue slices in rotatable hydrogel cylinders.
- Suspend samples in growth medium within light sheet microscopy chamber.
- Maintain controlled environment (37°C, 5% CO₂) throughout imaging.
- Acquire z-stack images at 15-30 minute intervals over 15-48 hours.
- Rotate sample 180° for multi-angle imaging to enhance spatial resolution.
- Process images using automated cell tracking algorithms.
- Correlate cell position data with morphological development of renal structures.
Technical Notes: Light sheet microscopy reduces photobleaching and phototoxic effects by several orders of magnitude compared to confocal microscopy, enabling truly dynamic assessment of MSC behavior within a renal microenvironment that closely mimics in vivo conditions [45].
Protocol: In Vivo RNA Dynamic Tracking (Dyna-vivo-seq)
Principle: This novel methodology combines in vivo metabolic RNA labeling with single-cell RNA sequencing to track transcriptional activity of engrafted MSCs and host renal cells simultaneously, providing insights into both localization and functional status [46].
Materials:
- 4-thiouridine (4sU) solution for intravenous injection
- Well-Paired-Seq2 (WPS2) platform
- Barcoded beads for single-cell capture
- Iodoacetamide (IAA) for chemical conversion
- Library preparation reagents
Procedure:
- Inject 4sU (biocompatible thymidine analog) via tail vein in mouse models.
- After 1.5-hour circulation, harvest kidney and dissociate into single-cell suspension.
- Pair single cells with barcoded beads in microwell chip.
- Lyse cells and capture mRNA on barcoded beads.
- Treat with IAA to convert 4sU to cytosine analog in newly synthesized RNAs.
- Perform reverse transcription, cDNA amplification, and library preparation.
- Sequence and analyze T-to-C conversions to distinguish new (MSC-influenced) from old RNA transcripts.
Technical Notes: Dyna-vivo-seq achieves a 20.7% new RNA labeling ratio with excellent biocompatibility and uniform distribution throughout renal tissue, enabling precise monitoring of transcriptional changes in different cell populations during AKI progression and recovery [46].
Signaling Pathways in MSC-Mediated Renal Repair
The therapeutic effects of retained MSCs in AKI are mediated through multiple interconnected signaling pathways that promote tissue repair and modulate the injury response.
Diagram 1: MSC Mechanisms in Renal Repair. This pathway illustrates how retained MSCs mediate tissue repair through paracrine signaling, mitochondrial transfer, and structural integration. The paracrine effects represent the primary mechanism, releasing factors that combat inflammation, apoptosis, oxidative stress, and promote angiogenesis [43] [11]. Mitochondrial transfer via tunneling nanotubes enhances cellular bioenergetics in injured renal cells [11], while direct integration contributes to structural restoration, particularly in chronic models [10].
Strategic Enhancement of MSC Retention and Efficacy
Several strategic approaches have been developed to address the critical challenge of low MSC retention in renal tissue, targeting different stages from preconditioning to delivery.
Diagram 2: MSC Retention Enhancement Strategies. This workflow outlines multidisciplinary approaches to improve MSC retention, including preconditioning to enhance homing capability [11], optimized delivery methods to maximize target tissue delivery [2], and microenvironment engineering to support cell survival and function post-engraftment [2].
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents for MSC Tracking Studies in Renal Tissue
| Reagent/Category | Specific Examples | Research Function |
|---|---|---|
| Cell Labeling | DiI, CM-DiI, GFP/Luciferase transduction | Fluorescent and bioluminescent tracking of MSC location and viability [10] [45] |
| Tracking Methodologies | Light sheet fluorescence microscopy, Multiphoton microscopy | Longitudinal, high-resolution 3D imaging in living tissues [47] [45] |
| RNA Dynamic Analysis | 4-thiouridine (4sU), Iodoacetamide (IAA) | Metabolic RNA labeling to distinguish new vs. old transcription [46] |
| Delivery Matrices | Alginate, Hyaluronic acid, Agarose hydrogels | 3D scaffolds for enhanced MSC retention and survival [2] |
| Preconditioning Agents | Chlorzoxazone, Atorvastatin, Hypoxic chambers | Enhancement of MSC therapeutic potency and homing capacity [11] |
Comprehensive cell tracking studies demonstrate that enhancing MSC retention in renal tissue is achievable through optimized delivery methods, particularly renal cortex injection, combined with strategic preconditioning and functional microenvironment support. The protocols outlined herein provide a standardized framework for quantifying and improving MSC engraftment, forming a critical methodological foundation for advancing paracrine-based therapy for AKI. Future directions should focus on clinical translation of these tracking technologies and retention enhancement strategies to bridge the current gap between preclinical efficacy and clinical outcomes.
Quantitative Data on Cell Dosing and Administration Routes
The therapeutic efficacy of Mesenchymal Stem Cell (MSC) therapy for Acute Kidney Injury (AKI) is highly dependent on both the administration route and the delivered cell number. The table below summarizes key quantitative findings from preclinical studies.
Table 1: Optimized MSC Dosing and Administration Routes in Preclinical AKI Models
| Administration Route | Animal Model | Optimal MSC Dose | Injection Volume | Key Efficacy Findings | Source |
|---|---|---|---|---|---|
| Renal Artery (RA) | Rat I/R Injury | 1x10⁵ cells | 500 µL | Most dramatic improvement in renal function & morphology; higher doses (1x10⁶) caused renal hypoperfusion. | [48] |
| Tail Vein (IV) | Mouse Cisplatin-AKI | 100 µg exosomes (from BMSCs) | Not Specified | Attenuated renal dysfunction and tubular injury in a dose-dependent manner. | [49] |
| Tail Vein (IV) | Rat Gentamicin-AKI | 1x10⁷ T-MSCs | 500 µL | Improved renal function, reduced apoptosis, and ameliorated oxidative stress. | [50] |
| Local Packing (Biological Membrane) | Mouse Glycerol-AKI | 1x10⁶ BM-MSCs | N/A (Packed on kidney) | Preserved renal function, ameliorated tubular lesions, and reduced apoptosis. | [51] |
Detailed Experimental Protocol: Renal Artery Injection in Rats
The following protocol, adapted from a study demonstrating the critical balance of cell dose and efficacy, details the steps for precise renal artery injection in a rat model of Ischemia-Reperfusion (I/R) injury [48].
Materials and Reagents
- Animals: Male Sprague-Dawley rats (250-300 g).
- MSCs: Bone-marrow derived MSCs, passages 6-8, labeled with CM-Dil cell tracker.
- Anesthetic: Pentobarbital sodium (40 mg/kg, intraperitoneal).
- Surgical Supplies: Microvascular clamps, 33-gauge needle (e.g., Cadence), micro-forceps, gelatin sponge.
- Cell Preparation: MSCs suspended in sterile Phosphate-Buffered Saline (PBS) at the required concentration (e.g., 1x10⁵ cells in 500 µL), kept on ice until infusion.
Procedure
Model Induction and Surgical Preparation:
- Anesthetize the rat and perform a midline abdominal incision.
- Perform a right nephrectomy.
- Induce ischemia by occluding the left renal pedicle with a non-traumatic microvascular clamp for 45 minutes.
- Visually confirm reperfusion upon clamp release.
Renal Artery Access:
- Carefully separate the left renal artery from surrounding connective tissue under a surgical microscope.
Sub-adventitial Puncture and Injection:
- Using micro-forceps for stabilization, puncture the renal artery at its proximal segment with a 33-gauge needle. First, enter the sub-adventitial level, then advance the needle through the arterial wall into the lumen.
- Slowly inject the prepared MSC suspension (500 µL) over 3-5 minutes to minimize hemodynamic shock and cell clumping.
Post-injection Hemostasis:
- Quickly withdraw the needle.
- Immediately apply a gelatin sponge over the puncture site to achieve hemostasis.
- Remove all surgical sponges from the abdominal cavity and close the incision in layers.
Key Considerations
- Dose Precision: This study highlights that the optimal therapeutic dose for renal artery injection is 1x10⁵ MSCs. A higher dose of 1x10⁶ MSCs led to renal hypoperfusion due to cell occlusion, worsening renal function [48].
- Injection Rate: A slow, controlled injection is critical to prevent embolic complications and allow for even distribution of cells.
Signaling Pathways in MSC-Mediated Renal Repair
MSCs exert their therapeutic effects primarily through paracrine actions. The following diagram illustrates key signaling pathways identified in the search results that are involved in MSC-mediated protection and repair in AKI.
The Scientist's Toolkit: Essential Research Reagents and Materials
The table below lists critical reagents and materials required for conducting experiments on renal cortex injection of MSCs for AKI, as derived from the cited protocols.
Table 2: Essential Research Reagents and Materials for MSC Renal Therapy Studies
| Item Name | Function/Application | Example from Search Results |
|---|---|---|
| CM-Dil Cell Tracker | Fluorescent labeling of MSCs for in vivo cell tracking and localization post-transplantation. | [48] |
| 33-Gauge Needle | Enables precise, minimally invasive puncture of the renal artery for local cell delivery. | [48] |
| Gelatin Sponge | Provides rapid hemostasis at the arterial puncture site following renal artery injection. | [48] |
| PKH26 Fluorescent Dye | Alternative lipophilic dye for stable, long-term cell membrane labeling and in vivo tracking. | [50] |
| Biological Membrane (e.g., Duragen) | A collagen-based scaffold used to pack and localize MSCs directly onto the surface of the injured kidney. | [51] |
| LY294002 (PI3K Inhibitor) | A specific inhibitor used in mechanistic studies to confirm the role of the PI3K/Akt pathway in MSC-mediated protection. | [51] |
| Exosome-Depleted FBS | Essential for preparing cell culture media intended for exosome isolation, preventing contaminating bovine vesicles. | [52] |
Within the broader research on renal cortex injection of mesenchymal stem cells (MSCs) for acute kidney injury (AKI) paracrine therapy, precise cell delivery is paramount for achieving therapeutic efficacy. The therapeutic potential of MSCs in AKI is largely attributed to their paracrine release of bioactive molecules, including cytokines, chemokines, growth factors, and extracellular vesicles, which collectively promote cell proliferation, inhibit apoptosis, enhance angiogenesis, and modulate immune responses [11] [2]. However, clinical translation is hampered by challenges such as low cell retention and poor survival of administered MSCs in the target tissue [11] [2]. Direct renal cortex injection has emerged as a local administration strategy to bypass the pulmonary first-pass effect associated with intravenous delivery, thereby increasing the engraftment of cells at the site of injury [2]. The integration of imaging guidance technologies, particularly ultrasound, is critical for executing this precise, localized delivery, ensuring that the therapeutic cells are deposited accurately within the renal cortex to maximize their paracrine benefits.
The Role of Imaging Guidance in MSCs Therapy for AKI
The Imperative for Precise Localization
The complex architecture of the kidney and the specific nature of ischemic or toxic injuries in AKI necessitate a targeted therapeutic approach. The renal cortex, which contains the glomeruli and proximal convoluted tubules, is often the primary site of injury in AKI. Systemically administered MSCs face significant obstacles, including entrapment in filtering organs like the lungs and liver, which drastically reduces the number of cells reaching the kidneys [2]. Local administration, specifically into the renal cortex, has been demonstrated in preclinical models to ameliorate these issues, leading to observed recovery of renal function and reduction in tubular injury [2]. Imaging guidance transforms this procedure from a blind injection into a precise, reproducible technique, allowing researchers to visually confirm the accurate placement of cells, minimize tissue trauma, and standardize protocols across experimental cohorts for reliable and comparable results.
Several imaging modalities can be employed for guiding interventional procedures. Their applicability depends on factors like resolution, penetration depth, cost, and real-time capability.
- Ultrasound (US): A predominant modality for real-time guidance due to its widespread availability, real-time imaging capability, lack of ionizing radiation, and relatively low cost. It provides excellent visualization of soft tissues and allows for dynamic tracking of the needle during injection procedures [53].
- Computed Tomography (CT): Offers high spatial resolution and detailed cross-sectional anatomical information. CT guidance is highly accurate for targeting specific locations and is often used for procedures requiring precise navigation through complex anatomical structures [53].
- Magnetic Resonance Imaging (MRI): Provides superior soft-tissue contrast without radiation exposure. While potentially offering the highest detail for distinguishing anatomical boundaries, its use is limited by higher costs, longer procedure times, and the need for specialized, MRI-compatible equipment.
For the specific application of renal cortex injection in rodent models, high-frequency ultrasound is often the most practical and effective modality due to its ability to provide real-time, high-resolution images of the kidney and the injection needle.
Application Note: Ultrasound-Guided Renal Cortex Injection Protocol
Experimental Workflow
The following diagram outlines the comprehensive workflow for preparing and performing an ultrasound-guided MSC injection into the renal cortex for AKI research.
Detailed Methodology
Objective: To deliver a precise volume of MSCs suspension directly into the renal cortex of a rodent AKI model using high-frequency ultrasound imaging for real-time guidance.
Materials and Equipment:
- Animal Model: Rodent (e.g., rat or mouse) with induced AKI (e.g., ischemia-reperfusion or gentamicin-induced) [3].
- MSCs Preparation: Harvested and characterized MSCs (e.g., bone marrow, umbilical cord, or tonsil-derived) in a sterile, single-cell suspension at a defined concentration (e.g., 1-10 x 10^6 cells/mL) in an appropriate vehicle like phosphate-buffered saline (PBS) [3] [2].
- Imaging System: High-frequency ultrasound imaging system (e.g., Vevo series) with a linear array transducer (e.g., 30-50 MHz).
- Injection Setup: Microsyringe (e.g., 50-100 µL Hamilton syringe) fitted with a fine-gauge needle (e.g., 30-33G).
- Anesthesia and Sterile Supplies: Isoflurane anesthetic system, hair clipper/depilatory cream, sterile surgical drapes, antiseptic solution, and eye ointment.
Pre-Procedure Preparation:
- MSCs Preparation: Prepare MSCs suspension. Keep on ice until immediately before injection. Gently mix before loading to ensure a homogeneous suspension.
- Animal Preparation: Anesthetize the animal using an isoflurane vaporizer. Secure the animal in a supine or lateral decubitus position. Apply eye ointment to prevent corneal drying. Remove fur from the abdominal area using clippers and/or depilatory cream. Thoroughly clean the skin with an antiseptic solution.
- Equipment Setup: Position the ultrasound probe securely on a stand. Apply a generous amount of acoustic coupling gel to the probe head. Load the MSCs suspension into the microsyringe, ensuring no air bubbles are present.
Step-by-Step Injection Procedure:
- Renal Localization: Gently place the ultrasound probe on the animal's abdomen. Identify the kidney in the longitudinal plane, recognizing its characteristic elliptical shape with a hyperechoic capsule and distinct corticomedullary differentiation.
- Needle Insertion: Under direct real-time ultrasound guidance, advance the injection needle at a shallow angle (approximately 20-30 degrees) towards the renal cortex. The needle will appear as a bright, hyperechoic line.
- Cortical Placement: Carefully advance the needle tip until it is positioned within the renal cortex. Confirm the tip location before proceeding.
- Cell Administration: Slowly depress the plunger to inject a controlled volume (e.g., 10-50 µL, depending on kidney size). A successful injection will often be visualized as a small, transient hypoechoic fluid bolus within the cortical tissue.
- Needle Withdrawal: Wait for 10-30 seconds after the injection to allow pressure to equalize and minimize backflow along the needle track. Slowly withdraw the needle.
Post-Procedure Care:
- Monitor the animal closely until it fully recovers from anesthesia on a warm pad.
- Administer post-operative analgesics as approved by the institutional animal care and use protocol.
Quantitative Data from Preclinical AKI Models
The table below summarizes key parameters and outcomes from selected preclinical studies utilizing localized MSC therapy in AKI, which can be used as a benchmark for designing your own experiments.
Table 1: Efficacy of MSCs Therapy in Preclinical AKI Models
| AKI Model / Species | MSC Source & Dose | Delivery Route | Key Functional Outcomes | Key Histological/Tissue Outcomes | Primary Cited Mechanisms |
|---|---|---|---|---|---|
| Gentamicin-induced / Rat [3] | Tonsil-derived (T-MSCs); 1x10^7 cells | Intravenous | ↓ BUN, ↓ SCr [3] | ↓ Tubular damage score, ↓ Apoptotic cells [3] | Anti-apoptotic, antioxidative effects, incorporation into tubules [3] |
| Ischemia/Reperfusion / Mouse [54] | Human Umbilical Cord (hUC-MSCs); 1x10^6 cells | Intravenous | ↓ SCr, ↓ BUN, Improved GFR [54] | ↓ ATN score, ↓ KIM-1 expression, ↓ TUNEL+ cells [54] | HA/CD44 binding, PI3K/AKT pathway activation [54] |
| STZ-induced Diabetic Kidney / Rat [55] | Bone Marrow (BM-MSCs); 2x10^6 cells | Intravenous | ↓ Urea, ↓ Creatinine [55] | ↓ KIM-1, ↓ NGAL, ↓ Fibrosis [55] | Suppression of PKC/NF-κB/STAT3 pathway [55] |
| Various AKI models / Review [2] | Various (BM, AD, UC); Multiple doses | Renal Cortex Injection | Renal function recovery [2] | Amelioration of tubular injury [2] | Enhanced engraftment, bypass of pulmonary trap [2] |
Abbreviations: BUN: Blood Urea Nitrogen; SCr: Serum Creatinine; GFR: Glomerular Filtration Rate; ATN: Acute Tubular Necrosis; KIM-1: Kidney Injury Molecule-1; NGAL: Neutrophil Gelatinase-Associated Lipocalin; TUNEL: Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling; HA: Hyaluronic Acid.
Signaling Pathways in MSC-Mediated Renal Repair
The therapeutic effects of MSCs are mediated through complex paracrine signaling that modulates multiple cellular pathways. The following diagram integrates key mechanisms identified in recent research.
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Materials for Renal Cortex Injection Experiments
| Item Category | Specific Examples & Specifications | Primary Function in Protocol |
|---|---|---|
| MSCs Sources | Bone Marrow-MSCs (BM-MSCs), Human Umbilical Cord-MSCs (hUC-MSCs), Tonsil-derived MSCs (T-MSCs), Adipose-derived MSCs (AD-MSCs) [11] [54] [3] | Therapeutic agent; source of paracrine factors (cytokines, exosomes) for renal repair. |
| Cell Culture Supplements | All-trans retinoic acid (ATRA) [54], Chlorzoxazone (CZ) [11], Hypoxic Preconditioning (1-5% O₂) [11] | Chemical or physical preconditioning to enhance MSC survival, anti-inflammatory phenotype, and paracrine function. |
| Vehicle Solution | Phosphate-Buffered Saline (PBS), Dulbecco's PBS (DPBS) | Isotonic solution for washing and resuspending MSCs for injection. |
| In Vivo Delivery Device | Hamilton Syringe (50-100 µL), 30-33G Fine-Gauge Needle | Precise delivery of a controlled cell suspension volume into the renal cortex with minimal tissue damage. |
| In Vivo Imaging System | High-Frequency Ultrasound (e.g., Vevo 3100) with 30-55 MHz transducer | Real-time, non-invasive visualization of kidney anatomy and needle trajectory for accurate injection guidance. |
| Animal Model Reagents | Gentamicin [3], Streptozotocin (STZ) [56] [55], Ischemia-Reperfusion Surgery | Induction of consistent and reproducible nephrotoxic, diabetic, or ischemic AKI models for therapeutic testing. |
| Assessment Kits | Serum Creatinine & BUN Assay Kits, KIM-1/NGAL ELISA Kits [3] [55], TUNEL Assay Kit [3] | Quantitative evaluation of renal functional impairment, specific kidney damage, and tubular cell apoptosis. |
Strategies to Enhance MSC Viability and Paracrine Function
Acute kidney injury (AKI) remains a worldwide public health issue with significant mortality and a lack of targeted pharmacological therapies [57]. Mesenchymal stem cell (MSC) therapy has emerged as a promising regenerative strategy for AKI, primarily mediated through the release of paracrine factors that exert anti-apoptotic, immunomodulatory, antioxidative, and pro-angiogenic effects [58]. However, the clinical translation of MSC-based therapies is hindered by critical limitations, including poor engraftment, low survival rates, and impaired paracrine ability of administered cells [57] [2] [58].
Preconditioning strategies have been developed to address these challenges by priming MSCs to enhance their therapeutic potential [58]. These approaches subject MSCs to sublethal stresses or expose them to specific bioactive molecules prior to administration, activating adaptive responses that improve their survival, migratory capacity, and paracrine function in the hostile microenvironment of injured renal tissue [57] [58]. For research focused on renal cortex injection of MSCs for AKI paracrine therapy, optimizing these preconditioning protocols is essential for maximizing therapeutic outcomes.
Preconditioning strategies can be broadly categorized into three primary modalities: hypoxia, chemical, and biological priming. The table below summarizes the key characteristics, mechanisms, and experimental support for each approach.
Table 1: Overview of Preconditioning Strategies for MSCs in AKI Research
| Preconditioning Strategy | Key Mechanistic Insights | Documented Effects on MSCs | In Vivo Evidence in AKI Models |
|---|---|---|---|
| Hypoxic Preconditioning | Upregulation of HIF-1α and CXCR4 [59]; Enhancement of renal tubular autophagy [60] | Improved migration and retention [59]; Enhanced anti-oxidative properties [60]; Increased secretion of beneficial trophic factors [60] | Reduced kidney function decline in rat I/R model [60]; Enhanced functional recovery in ischemic AKI [59] |
| Chemical Preconditioning | Cobalt chloride as hypoxia mimetic [59] | Increased HIF-1α and CXCR4 expression; Enhanced migration capacity [59] | Improved recruitment to ischemic kidney; Reduced kidney injury in rat I/R model [59] |
| Biological Preconditioning (Cytokine/GF Priming) | Strategic activation of specific receptor-mediated signaling pathways (e.g., CXCR4) [59] | Enhanced homing capacity; Potentiated paracrine activity [58] [59] | Data specifically from cytokine-preconditioned MSCs in AKI models is an active research area [58] |
Detailed Experimental Protocols for Preconditioning Strategies
Hypoxic Preconditioning Protocol
Objective: To enhance the migratory capacity and paracrine function of MSCs through controlled hypoxic exposure prior to renal cortex injection.
Materials:
- Mesenchymal stem cells (bone marrow-derived recommended)
- Hypoxia chamber or multi-gas CO₂ incubator capable of maintaining 1% O₂
- Complete culture medium (α-MEM supplemented with 16.6% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine)
- Trypsin/EDTA solution for cell detachment
Procedure:
- Culture MSCs under standard conditions (21% O₂, 5% CO₂, 74% N₂) until 70-80% confluence.
- Passage cells using standard techniques and seed at a density of 5,000 cells/cm² in complete medium.
- Place cells in a hypoxic chamber with precisely controlled atmosphere (1% O₂, 5% CO₂, 94% N₂) maintained by a compact gas oxygen controller.
- Culture cells under hypoxic conditions for 24-48 hours. The optimal duration may vary by cell source and should be determined empirically.
- Harvest preconditioned MSCs using trypsin/EDTA and resuspend in appropriate injection buffer for renal cortex delivery.
- Validate preconditioning efficacy by assessing HIF-1α and CXCR4 expression via Western blot or flow cytometry [60] [59].
Quality Control: Monitor cell viability post-preconditioning using trypan blue exclusion, ensuring >95% viability before transplantation.
Chemical Preconditioning with Cobalt Chloride
Objective: To mimic hypoxic preconditioning using cobalt chloride for enhanced CXCR4 expression and migration capacity.
Materials:
- MSCs at passage 3-5
- Cobalt chloride (CoCl₂) stock solution (200 mM in PBS)
- Complete culture medium
- siRNA targeting HIF-1α (for mechanistic studies)
Procedure:
- Culture MSCs to 70% confluence in standard conditions.
- Prepare preconditioning medium by adding CoCl₂ to complete culture medium at a final concentration of 200 μmol/L.
- Replace normal culture medium with CoCl₂-containing medium and incubate cells for 24 hours.
- Harvest preconditioned MSCs and wash twice with PBS to remove residual cobalt.
- For renal cortex injection, resuspend cells at appropriate concentration in sterile saline.
- For validation: Assess HIF-1α and CXCR4 upregulation at mRNA and protein levels using real-time RT-PCR and Western blot [59].
Mechanistic Investigation: To confirm HIF-1α dependence, transfert MSCs with HIF-1α siRNA prior to cobalt treatment, which should abrogate the enhancement of CXCR4 expression and migration capacity [59].
Biological Preconditioning with Cytokines
Objective: To prime MSCs with specific cytokines to enhance homing capacity and paracrine function.
Materials:
- Recombinant human SDF-1α/CXCL12
- Serum-free MSC medium
- Migration assay reagents (Transwell plates, appropriate membranes)
Procedure:
- Culture MSCs to 70% confluence.
- Pre-treat MSCs with SDF-1α (50-100 ng/mL) in serum-free medium for 12-24 hours.
- Alternatively, consider other cytokines based on target pathways (e.g., TGF-β, TNF-α at sublethal concentrations).
- Harvest cells for injection or assess migration enhancement through Transwell assay toward an SDF-1α gradient.
- For in vivo studies, inject preconditioned MSCs via renal cortex route and track retention and distribution.
Validation: Confirm enhanced migration through scratch-wounding healing assay or Transwell migration assay [59].
Quantitative Analysis of Preconditioning Effects
The efficacy of preconditioning strategies is quantified through specific experimental parameters. The table below summarizes documented quantitative effects from preclinical studies.
Table 2: Quantitative Effects of Preconditioning Strategies on MSC Function
| Parameter Measured | Preconditioning Method | Experimental Findings | Research Context |
|---|---|---|---|
| Migration Capacity | Hypoxic mimetic (CoCl₂) | Healing rate increased from 38.2% to 71.4% (scratch assay) [59] | In vitro MSC culture |
| Gene Expression | Hypoxic mimetic (CoCl₂) | Significant increase in HIF-1α and CXCR4 mRNA and protein [59] | In vitro MSC analysis |
| In vivo Retention | Hypoxic mimetic (CoCl₂) | Greater migration and longer retention in ischemic kidneys vs. normoxic MSCs [59] | Rat I/R AKI model |
| Renal Function | HMSCs | Attenuated kidney function decline in I/R injury [60] | Rat I/R AKI model |
| Tubular Autophagy | HMSCs | Upregulation of LC3B, Atg5, and Beclin 1 in renal tubular cells [60] | In vivo and in vitro models |
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagents for Preconditioning Studies
| Reagent/Category | Specific Examples | Research Function | Application Notes |
|---|---|---|---|
| Hypoxia-Inducing Agents | Cobalt chloride (CoCl₂) [59] | HIF-1α stabilizer; induces hypoxic response normoxically | Use at 200 μmol/L for 24h; validate HIF-1α upregulation |
| CXCR4 Modulators | AMD3100 (CXCR4 antagonist) [59] | Mechanistic studies of SDF-1/CXCR4 axis in MSC migration | Use to confirm CXCR4-dependent migration effects |
| siRNA Tools | HIF-1α siRNA [59] | Gene-specific knockdown to establish mechanism | Confirm HIF-1α dependence in preconditioning strategies |
| Cell Labeling Agents | SPIO nanoparticles, CM-DiL fluorescent dye [59] | Cell tracking for in vivo migration and retention studies | SPIO enables MR imaging; CM-DiL enables fluorescence detection |
| Autophagy Modulators | 3-methyladenine (autophagy inhibitor) [60] | Mechanistic studies on autophagy-mediated renoprotection | Use to validate role of autophagy in MSC therapeutic effects |
Signaling Pathways and Experimental Workflows
Hypoxic Preconditioning Signaling Pathway
Experimental Workflow for Preconditioning Studies
The preconditioning approaches detailed in these application notes provide robust methodologies for enhancing the therapeutic potential of MSCs in AKI research, particularly in the context of renal cortex injection. The strategic implementation of hypoxic, chemical, or biological priming can significantly improve key parameters for successful paracrine therapy, including cellular migration, retention within target tissue, and secretion of beneficial factors.
For research applications, we recommend initial validation of preconditioning efficacy through in vitro migration assays and expression analysis of key markers (HIF-1α, CXCR4) before proceeding to in vivo studies. The renal cortex injection route provides direct access to the site of injury, maximizing the benefits of preconditioned MSCs by bypassing systemic trafficking barriers. When designing preclinical studies, consider incorporating multiple assessment timepoints to evaluate both short-term retention and long-term functional outcomes.
The therapeutic potential of Mesenchymal Stem Cells (MSCs) for Acute Kidney Injury (AKI) is primarily mediated through their paracrine secretion of bioactive molecules, including growth factors, cytokines, and extracellular vesicles, which promote tissue repair, modulate immune responses, and inhibit apoptosis and fibrosis [11] [35]. However, the clinical translation of MSC-based therapies is significantly hampered by the poor survival, low retention, and reduced paracrine function of MSCs following transplantation into the hostile microenvironment of injured renal tissue [11] [35]. Conventional two-dimensional (2D) monolayer culture systems fail to replicate the complex physiological cell-cell and cell-matrix interactions, leading to MSCs that quickly undergo senescence and exhibit altered morphology, decreased stemness, and impaired therapeutic function [35] [61] [62].
To address these limitations, three-dimensional (3D) culture systems have emerged as a transformative technology. By recapitulating a more physiologically relevant microenvironment, 3D culture systems bridge the gap between traditional in vitro models and the complex in vivo architecture of living tissues [35] [61]. This application note details the use of spheroids (scaffold-free) and hydrogels (scaffold-based) as advanced 3D culture methodologies to enhance the functionality, viability, and paracrine activity of MSCs, specifically within the context of renal cortex injection for AKI paracrine therapy research.
Comparative Analysis of 2D vs. 3D Culture Systems for MSCs
Transitioning from 2D to 3D culture fundamentally alters MSC morphology, proliferation, and, most importantly, their secretory profile. The table below summarizes the key functional differences that impact therapeutic efficacy in AKI.
Table 1: Functional Comparison of 2D vs. 3D MSC Culture Systems
| Parameter | 2D Monolayer Culture | 3D Culture (Spheroids/Hydrogels) | Impact on AKI Therapy |
|---|---|---|---|
| Cell Morphology | Flattened, elongated morphology [61] | Compact, spherical or matrix-embedded structure mimicking native tissue architecture [35] | Promotes a more physiological cell state, enhancing relevant paracrine signaling [35]. |
| Proliferation & Senescence | Rapid proliferation followed by accelerated senescence and loss of function [35] [62] | Reduced proliferation rates but enhanced cell viability and resistance to senescence [35] [62] | Increases the delivery of viable, functionally active MSCs to the injured renal cortex [35]. |
| Paracrine Secretion | Altered gene/protein expression; suboptimal secretion of therapeutic factors [11] [61] | Enhanced secretion of angiogenic (VEGF, HGF), anti-inflammatory, and anti-apoptotic factors [11] [35] | Directly improves the capacity for renal repair, vascularization, and immunomodulation in AKI [11]. |
| In Vivo Survival Post-Transplantation | Poor survival and engraftment in the hostile AKI microenvironment [11] [35] | Improved retention and survival upon renal cortex injection due to protective 3D matrix [35] | Extends the duration of paracrine activity at the site of injury, leading to more durable therapeutic effects [35]. |
3D Culture Methodologies: Spheroids and Hydrogels
Scaffold-Free 3D Culture: Spheroids
Spheroid culture relies on cell aggregation to form dense, multicellular structures, restoring critical cell-cell contacts and creating endogenous extracellular matrix.
Protocol 3.1.1: Generation of MSC Spheroids via the Hanging Drop Method
- Objective: To generate uniform, scaffold-free MSC spheroids for renal therapy research.
- Materials:
- Research Reagent: Phosphate-Buffered Saline (PBS)
- Research Reagent: DMEM/F12 culture medium, supplemented with 10% FBS and 1% Penicillin-Streptomycin
- Equipment: Sterile, low-adhesion Petri dishes or 96-well round-bottom ultra-low attachment (ULA) plates
- Method:
- Cell Preparation: Harvest MSCs (e.g., human Umbilical Cord-derived MSCs, hUCMSCs) from 2D culture using standard trypsinization. Create a single-cell suspension and centrifuge at 300 x g for 5 minutes.
- Resuspension: Resuspend the cell pellet in complete culture medium to a final concentration of 1-2 x 10^6 cells/mL.
- Hanging Drop Formation:
- Pipette 20-30 µL droplets of the cell suspension onto the lid of a sterile Petri dish.
- Carefully invert the lid and place it over the bottom of the dish, which is filled with PBS to maintain humidity.
- Incubation: Culture the cells for 48-72 hours in a standard incubator (37°C, 5% CO₂).
- Harvesting: After 72 hours, spherical aggregates will have formed. Gently pipette the spheroids from the hanging drops for downstream applications or analysis.
- Alternative Method: For higher throughput, the cell suspension can be seeded into 96-well ULA plates. The well geometry forces cells to aggregate at the bottom, forming a single spheroid per well.
Scaffold-Based 3D Culture: Hydrogels
Hydrogels are water-swollen, porous polymeric networks that serve as an artificial extracellular matrix (ECM), providing mechanical support and biochemical cues [35].
Protocol 3.2.1: Encapsulation of MSCs in Hydrogels for 3D Culture
- Objective: To encapsulate MSCs within a hydrogel scaffold to mimic the renal ECM and enhance MSC function.
- Materials:
- Research Reagent: Matrigel (Basement Membrane Extract), a gold-standard, animal-derived hydrogel rich in laminin, collagen IV, and growth factors [61].
- Research Reagent: GrowDex (Nanofibrillar Cellulose), a defined, plant-based, biologically inert hydrogel [61].
- Research Reagent: Pre-chilled (4°C) pipette tips and culture plates.
- Method:
- Cell Preparation: Harvest and count MSCs as in Protocol 3.1.1. Keep the cell pellet on ice.
- Hydrogel Preparation: Thaw Matrigel or prepare GrowDex on ice to prevent premature polymerization.
- Mixing: Gently mix the concentrated MSC pellet with the cold hydrogel to achieve a final density of 1-5 x 10^6 cells/mL in the hydrogel. Avoid introducing air bubbles.
- Polymerization:
- For the Sandwich Method, pipette a small volume (e.g., 50 µL) of the cell-hydrogel mixture into the center of a multiwell plate and spread gently. Incubate for 20-30 minutes at 37°C to allow gelation, then carefully overlay with complete culture medium.
- For the Mini-dome Method, pipette a larger drop (e.g., 100-200 µL) directly onto the plate surface, forming a "dome." Incubate to set before adding culture medium.
- Culture and Monitoring: Culture the encapsulated MSCs, changing the medium every 2-3 days. Monitor cell viability and morphology using microscopy.
Table 2: Comparison of Common Hydrogel Scaffolds for MSC Culture
| Hydrogel Type | Composition | Key Advantages | Key Limitations | Suitability for AKI MSC Research |
|---|---|---|---|---|
| Matrigel | Basement membrane extract from mouse sarcoma; contains laminin, collagen IV, entactin, growth factors [61] | Promotes robust spheroid formation and cell differentiation; high bioactivity [61] | Complex, undefined composition; high batch-to-batch variability; animal-derived [61] | Excellent for fundamental studies requiring high bioactivity. |
| GelTrex | Reduced-growth factor basement membrane extract [61] | More standardized than Matrigel; reduced growth factor content [61] | Still animal-derived and subject to variability [61] | Suitable for applications where defined growth factor levels are desired. |
| GrowDex | Nanofibrillar cellulose from birch trees [61] | Defined, plant-based composition; low batch-to-batch variability; biologically inert [61] | Lacks inherent biological cues, which may require functionalization [61] | Ideal for controlled, reproducible studies and as a delivery vehicle. |
Experimental Workflow and Signaling Pathways
The following diagram illustrates the integrated experimental workflow from 3D culture establishment to functional analysis in a preclinical AKI model.
Diagram 1: 3D MSC Culture to AKI Model Workflow.
Enhanced MSC functionality within 3D cultures activates key signaling pathways that drive renal repair. The core pathway is centered on Hypoxia-Inducible Factor 1-alpha (HIF-1α), which is stabilized under the mild hypoxic conditions within spheroids and hydrogels.
Diagram 2: HIF-1α-Mediated Paracrine Pathway in 3D MSCs.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Research Reagent Solutions for 3D MSC Culture in AKI Research
| Research Reagent / Material | Function and Application |
|---|---|
| Ultra-Low Attachment (ULA) Plates | Prevents cell adhesion, forcing aggregation into scaffold-free spheroids. Essential for high-throughput spheroid production [35]. |
| Matrigel / GelTrex | Animal-derived hydrogel scaffolds providing a bioactive basement membrane environment to support MSC 3D growth and enhance paracrine function [61]. |
| GrowDex | Plant-based, defined hydrogel offering a reproducible and inert scaffold for MSC encapsulation, ideal for delivery vehicle studies [61]. |
| Chlorzoxazone (CZ) | FDA-approved drug used for chemical preconditioning of MSCs. Induces an anti-inflammatory phenotype, boosting immunosuppressive capacity for AKI therapy [11]. |
| Hypoxic Chamber (1-5% O₂) | System for physical preconditioning of MSCs. Mimics the ischemic kidney environment, enhancing MSC survival, HIF-1α pathway activation, and therapeutic factor secretion [11]. |
The adoption of 3D culture systems, specifically spheroids and hydrogels, represents a critical advancement in MSC-based therapy development for Acute Kidney Injury. These methodologies directly address the core limitations of traditional 2D culture by generating MSCs with enhanced paracrine function, improved resilience, and greater in vivo survival. The protocols and analyses provided herein offer a robust framework for researchers to implement these technologies, thereby accelerating the translation of more effective and reliable MSC therapies from the bench to the bedside for the treatment of AKI.
The administration of mesenchymal stem cells (MSCs) via renal cortex injection represents a promising therapeutic strategy for Acute Kidney Injury (AKI), primarily leveraging their paracrine activity rather than their differentiation potential [63]. These cells naturally secrete a diverse array of therapeutic factors—including growth factors, cytokines, and chemokines—that promote tissue repair, modulate immune responses, and inhibit fibrosis [11] [63]. However, a significant limitation hindering the clinical translation of this approach is the suboptimal secretion profile of naive MSCs within the harsh, inflammatory microenvironment of the injured kidney [11]. Genetic engineering emerges as a powerful tool to overcome this hurdle by enhancing the production and secretion of key therapeutic factors, thereby augmenting the efficacy and reliability of MSC-based paracrine therapy for AKI.
Key Therapeutic Factors for AKI and Engineering Targets
The protective effects of MSCs in AKI are largely mediated by their secretome. Research has identified several crucial factors that contribute to renal repair, making them prime targets for genetic enhancement.
Table 1: Key Therapeutic Factors Secreted by MSCs and Their Roles in AKI
| Therapeutic Factor | Full Name | Primary Role in AKI Repair | Evidence |
|---|---|---|---|
| HO-1 | Heme Oxygenase-1 | Confers anti-apoptotic, anti-inflammatory, and pro-angiogenic properties; essential for MSC-mediated protection [64]. | |
| VEGF-A | Vascular Endothelial Growth Factor A | Promotes angiogenesis and vascular repair, improving blood flow to damaged renal tissue [64] [15]. | |
| HGF | Hepatocyte Growth Factor | Exhibits anti-fibrotic and regenerative effects, supporting tubular cell survival and proliferation [64] [15]. | |
| SDF-1 | Stromal Cell-Derived Factor-1 | Facilitates homing of progenitor cells to sites of injury and contributes to tissue repair processes [64]. | |
| BMP-7 | Bone Morphogenetic Protein-7 | Ameliorates glomerular fibrosis, counteracting the progression to chronic kidney disease [15]. | |
| FGF-2 | Fibroblast Growth Factor-2 | Supports cell proliferation, tissue repair, and angiogenesis [15]. |
The importance of these factors is underscored by studies showing that MSCs lacking specific genes, such as HO-1, lose their protective capabilities. HO-1-deficient MSCs demonstrate reduced expression and secretion of SDF-1, VEGF-A, and HGF, and their conditioned medium fails to rescue functional and morphological changes in cisplatin-induced AKI [64]. This evidence solidifies HO-1 and other key factors as high-priority targets for genetic intervention.
Protocol: Genetic Modification of MSCs to Enhance Secretory Profile
This protocol details a methodology to genetically engineer human bone marrow-derived MSCs (hBM-MSCs) to overexpress Heme Oxygenase-1 (HO-1), a critical therapeutic factor for AKI.
Materials and Reagents
- Cells: Primary human BM-MSCs (commercially available or isolated from donor marrow).
- Culture Medium: Dulbecco's Modified Eagle Medium (DMEM) with low glucose, supplemented with 10% Fetal Bovine Serum (FBS) and 1% Penicillin-Streptomycin.
- Plasmid Vector: Lentiviral expression vector (e.g., pLVX-EF1α) containing the human HMOX1 (HO-1) coding sequence.
- Packaging Plasmids: psPAX2 and pMD2.G for lentivirus production.
- Transfection Reagent: Polyethylenimine (PEI).
- Target Cells: HEK293T cells for virus production.
- Selection Antibiotic: Puromycin.
- Buffers: Phosphate-Buffered Saline (PBS), Trypsin-EDTA solution.
Step-by-Step Procedure
Part A: Production of Lentiviral Particles
- Cell Seeding: Seed HEK293T cells in a 10 cm culture dish at 70-80% confluency in complete DMEM medium without antibiotics.
- Plasmid Transfection: For one dish, prepare a DNA mixture containing 10 µg of the pLVX-HO-1 plasmid, 7.5 µg of the psPAX2 packaging plasmid, and 2.5 µg of the pMD2.G envelope plasmid in 500 µL of sterile, serum-free DMEM. In a separate tube, dilute 30 µL of PEI transfection reagent in 500 µL of serum-free DMEM. Combine the two solutions, vortex briefly, and incubate for 15-20 minutes at room temperature to form DNA-PEI complexes.
- Transfection: Add the DNA-PEI complex mixture dropwise to the HEK293T cells. Gently swirl the dish to ensure even distribution.
- Medium Change: After 6-8 hours, carefully replace the transfection medium with 10 mL of fresh complete medium.
- Virus Harvest: At 48 and 72 hours post-transfection, collect the culture supernatant containing the lentiviral particles. Centrifuge the supernatant at 500 × g for 10 minutes to remove cell debris. Filter the supernatant through a 0.45 µm PVDF filter. Aliquot and store the viral supernatant at -80°C.
Part B: Transduction of hBM-MSCs
- Cell Preparation: Seed early-passage (P3-P5) hBM-MSCs in a 6-well plate at a density of 1 × 10^5 cells per well. Culture until they reach 50-60% confluency.
- Transduction: Thaw the viral supernatant quickly at 37°C. Replace the culture medium on the hBM-MSCs with the viral supernatant, supplemented with 8 µg/mL polybrene to enhance transduction efficiency.
- Incubation: Incubate the cells with the viral particles for 24 hours.
- Recovery: After 24 hours, carefully remove the viral-containing medium and replace it with fresh, complete MSC culture medium.
- Selection: Begin antibiotic selection with 1-2 µg/mL puromycin 48 hours post-transduction. Maintain the selection pressure for at least 7 days, until all non-transduced control cells have died.
Part C: Validation of Genetically Engineered MSCs (HO-1-MSCs)
- Functional Validation (In Vitro):
- Western Blot: Confirm HO-1 protein overexpression by lysing cells and subjecting the proteins to SDS-PAGE, followed by immunoblotting with an anti-HO-1 antibody [64].
- ELISA: Quantify the secretion of HO-1 and associated factors (VEGF-A, HGF) in the conditioned medium of HO-1-MSCs compared to control MSCs using commercial ELISA kits [64].
- Conditioned Medium Rescue Assay: Treat a cisplatin-injured renal tubular cell line (e.g., HK-2) with conditioned medium from either HO-1-MSCs or control MSCs. Assess functional and morphological changes, including apoptosis (e.g., by cleaved caspase-3 western blot) and cell viability [64].
Signaling Pathways in Engineered MSC Therapy
The therapeutic effect of genetically enhanced MSCs is mediated through complex signaling pathways that modulate the renal microenvironment. The following diagram illustrates the key pathway by which HO-1-overexpressing MSCs confer protection.
Diagram 1: The protective paracrine pathway initiated by HO-1-engineered MSCs. HO-1 overexpression enhances the secretion of key factors that act on the injured kidney, promoting an anti-inflammatory macrophage phenotype and ultimately leading to tissue repair.
Furthermore, the secretome from MSCs, including extracellular vesicles, can act on specific immune cells in the kidney. Adipose-derived MSC-EVs (AMSC-EVs), for example, have been shown to promote the polarization of renal CX3CR1+ macrophages towards the reparative M2 phenotype by suppressing the TXNIP-IKKα/NF-κB signaling pathway, thereby altering the inflammatory microenvironment and facilitating recovery [12].
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents for Genetic Engineering and Validation of MSCs for AKI Research
| Reagent / Tool | Function / Application | Example / Note |
|---|---|---|
| Lentiviral Vector System | Stable gene delivery into MSCs for long-term overexpression of target genes (e.g., HO-1). | pLVX-EF1α vector system; allows for strong, constitutive expression. |
| Polyethylenimine (PEI) | A cost-effective transfection reagent for producing lentiviral particles in HEK293T cells. | Linear PEI (MW 25,000) is commonly used. |
| Puromycin | A selection antibiotic for enriching successfully transduced MSCs. | A kill curve must be established to determine the optimal concentration for your MSC line. |
| ELISA Kits | Quantitative measurement of secreted therapeutic factors (VEGF, HGF, SDF-1) in conditioned medium. | Commercial kits available from R&D Systems, etc. [64]. |
| Anti-HO-1 Antibody | Validation of HO-1 protein overexpression via Western Blot analysis. | Antibodies are available from suppliers like Stressgen [64]. |
| Cisplatin | Induction of a well-characterized nephrotoxic AKI model in vitro (on tubular cells) and in vivo (in mice). | Enables functional testing of engineered MSC efficacy [64] [12]. |
| CX3CR1-Cre; Rosa26-LSL-DTR Mice | A transgenic model for specific ablation of CX3CR1+ macrophages to study their role in MSC-EV mediated therapy. | Critical for mechanistic studies in vivo [12]. |
Genetic engineering of MSCs to enhance their secretion of therapeutic factors represents a cutting-edge strategy to potentiate cell-based paracrine therapy for AKI. By focusing on key targets like HO-1, researchers can develop more potent and reliable "off-the-shelf" MSC products. The protocols and tools outlined herein provide a roadmap for developing, validating, and mechanistically understanding the function of these engineered cells, ultimately accelerating their translation towards clinical application for a condition that currently lacks effective pharmacological treatments.
The administration of mesenchymal stem cells (MSCs) via renal cortex injection represents a promising therapeutic strategy for Acute Kidney Injury (AKI), primarily leveraging their paracrine activity rather than direct differentiation. The efficacy of this approach is often limited by poor cell retention and survival at the injury site. Biomaterial-assisted delivery systems are engineered to overcome these hurdles by providing a protective, three-dimensional niche that enhances MSC viability and facilitates the sustained, localized release of paracrine factors, such as growth factors, cytokines, and extracellular vesicles. This protocol details the application of hydrogels and decellularized extracellular matrix (ECM)-based scaffolds for this purpose, framed within preclinical research on AKI [65] [66] [11].
Key Biomaterial Platforms for MSC Delivery
The table below summarizes the primary biomaterial scaffolds used for sustaining MSC paracrine release in renal therapy.
Table 1: Biomaterial Scaffolds for Sustained Paracrine Release
| Biomaterial Platform | Composition | Key Properties | Mechanism of Sustained Release | Application in AKI Research |
|---|---|---|---|---|
| Natural Polymer Hydrogels (e.g., Collagen, Alginate) | Organic, bio-based materials (proteins, polysaccharides) [65]. | High biocompatibility, biodegradability, low immunogenicity, mimic native ECM [65]. | Encapsulates MSCs and their secreted factors; diffusion-controlled release tuned by hydrogel cross-linking density and porosity [11]. | Injected directly into the renal cortex; provides a temporary 3D niche supporting MSC survival and paracrine function [11]. |
| Synthetic Polymer Hydrogels (e.g., PEG, PLGA) | FDA-approved organic polymers like Polyethylene Glycol (PEG) and Poly(lactic-co-glycolic acid) (PLGA) [65]. | Precisely tunable mechanical properties, degradation rates, and easy large-scale manufacture [65]. | Release kinetics engineered via polymer chemistry (e.g., chain length, hydrolytic degradation) [65]. | Used as injectable carriers for MSCs; functionalization with adhesion peptides (e.g., RGD) can enhance cell-matrix interactions [11]. |
| Decellularized Renal ECM Scaffolds | Bio-based material derived from acellular kidney tissue, preserving native collagen, laminin, and fibronectin [67]. | Maintains organ-specific micro-architecture and biochemical cues; inherently biocompatible and pro-regenerative [67]. | The natural ECM composition binds and sequesters bioactive factors, facilitating their controlled presentation to surrounding tissue [67]. | Implanted as a patch at the site of injury; can be pre-seeded with MSCs to create a bioengineered graft [67]. |
Experimental Protocol: Renal Cortex Injection of MSC-Laden Hydrogels
This protocol outlines the preparation of an MSC-laden hydrogel and its injection into the renal cortex of a rodent AKI model.
Materials and Reagents
Table 2: Research Reagent Solutions
| Item | Function/Description |
|---|---|
| Mesenchymal Stem Cells (MSCs) | Primary therapeutic agent; source can be bone marrow, umbilical cord, or adipose tissue [68] [66]. |
| Hydrogel Precursor (e.g., Methacrylated Alginate) | Forms the 3D scaffold for cell encapsulation upon cross-linking [11]. |
| Cross-linking Initiator (e.g., CaSO₄ slurry or UV light with photoinitiator) | Triggers the gelation process to form a solid hydrogel from the precursor solution [11]. |
| Platelet-Rich Plasma (PRP) | A biological preconditioning agent; contains growth factors (PDGF, VEGF, TGF-β) to enhance MSC proliferation and paracrine activity [68]. |
| Dulbecco's Modified Eagle Medium (DMEM) | Culture medium for MSC expansion and hydrogel preparation [68]. |
| Animal Model (e.g., Rat Glycerin-Induced AKI) | A standard preclinical model for testing therapeutic efficacy of MSC therapies for AKI [68]. |
Detailed Methodology
Part A: Preconditioning and Preparation of MSCs
- Cell Culture: Isolate and culture human umbilical cord MSCs (hucMSCs) in DMEM supplemented with 10% FBS under standard conditions (37°C, 5% CO₂) [68].
- Preconditioning (Optional but Recommended): To enhance therapeutic potency, precondition MSCs by incubating them with PRP medium (containing 1×10⁸ platelets/mL) for 12 hours. Replace with fresh culture medium for 24 hours prior to encapsulation. This activates the AKT/Rab27 pathway, boosting exosome secretion [68].
- Harvesting: At 80-90% confluence, wash cells with PBS, detach using trypsin/EDTA, and centrifuge to form a cell pellet.
Part B: Preparation of MSC-Laden Hydrogel
- Hydrogel Solution: Dissolve the sterile hydrogel precursor (e.g., 2% w/v methacrylated alginate) in physiological buffer.
- Cell Encapsulation: Resuspend the MSC pellet in the hydrogel solution to a final concentration of 5-10 × 10⁶ cells/mL. Mix gently to ensure a uniform cell suspension.
- Loading into Syringe: Draw the cell-polymer mixture into a 1mL sterile syringe. Avoid creating bubbles.
- Cross-linking (In-Situ Gelation): Attach a fine-gauge needle (e.g., 27G). For ionic cross-linking (e.g., alginate with Ca²⁺), the syringe can be connected to a second syringe containing the cross-linker solution via a connector, allowing mixing and gelation just before ejection.
Part C: Surgical Renal Cortex Injection
- AKI Model and Anesthesia: Induce AKI in male Sprague-Dawley rats (180-200 g) via intramuscular injection of 50% glycerin (10 mL/kg) [68]. After 24 hours, anesthetize the animal and secure it in a lateral position.
- Surgical Exposure: Make a lateral incision on the dorsal side to expose the kidney. Gently isolate the kidney from surrounding adipose tissue.
- Injection Procedure: Using a microsyringe pump or manual steady pressure, slowly inject 20-50 µL of the MSC-hydrogel composite at 2-3 different sites within the renal cortex. To minimize reflux, hold the needle in place for 60 seconds post-injection before slowly withdrawing it [11].
- Closure and Recovery: Return the kidney to the renal fossa and suture the muscle and skin layers. Monitor animals until they recover from anesthesia.
Part D: Post-Injection Analysis
- Functional Assessment: At designated endpoints (e.g., day 3 post-injection), collect blood samples to measure serum creatinine and Blood Urea Nitrogen (BUN) levels to assess renal function recovery [68].
- Histological Analysis: Harvest kidney tissue, fix in 4% paraformaldehyde, and embed in paraffin. Section and stain with Hematoxylin and Eosin (H&E) to observe histopathological changes. Use TUNEL staining to quantify tubular cell apoptosis [68].
- Paracrine Factor Tracking: To confirm the sustained release and action of MSC-derived exosomes, isolate exosomes from preconditioned MSCs and label them with a lipophilic dye (e.g., CM-DiR) before encapsulation. Track their retention and distribution in the kidney using an in vivo imaging system (IVIS) [68].
Signaling Pathways and Experimental Workflow
The following diagrams illustrate the key molecular mechanism enhanced by preconditioning and the overall experimental workflow.
Diagram 1: MSC Paracrine Activation via PRP
Diagram 2: Experimental Workflow for Renal Injection
The therapeutic potential of mesenchymal stem cell (MSC) transplantation for acute kidney injury (AKI) primarily arises from their paracrine activity rather than direct differentiation into renal cells [11] [69]. These paracrine effects include immunomodulation, anti-apoptosis, anti-fibrosis, and promotion of angiogenesis [11] [20]. However, the hostile microenvironment of injured renal tissue—characterized by inflammation, oxidative stress, and fibrosis—significantly limits the survival, retention, and secretory function of administered MSCs [11] [70]. To overcome these limitations, combination strategies that pair MSC therapy with pharmacological agents have emerged as a promising approach to enhance therapeutic efficacy [11] [70]. These synergistic combinations work through multiple mechanisms: directly enhancing MSC functionality via preconditioning, improving the renal microenvironment to support MSC survival and function, and providing complementary therapeutic actions that target different aspects of the AKI pathophysiology [71] [70]. This application note details specific pharmacological agents, their mechanisms of action, and experimental protocols for developing effective MSC-drug combination therapies for AKI.
Pharmacological Agents for MSC Combination Therapy
The table below summarizes well-researched pharmacological agents that demonstrate synergistic effects when combined with MSC therapy for kidney injury.
Table 1: Pharmacological Agents for MSC Combination Therapy in AKI
| Pharmacological Agent | Class / Properties | Synergistic Mechanism with MSCs | Experimental Evidence |
|---|---|---|---|
| Chlorzoxazone (CZ) [11] | FDA-approved muscle relaxant | Induces anti-inflammatory phenotype in MSCs; enhances secretion of immunomodulatory factors [11]. | Attenuated renal inflammation and glomerular fibrinoid necrosis in Thy1.1 antibody-induced AKI model [11]. |
| Atorvastatin (Ator) [11] | Statin (HMG-CoA reductase inhibitor); anti-apoptotic, antioxidant, anti-inflammatory | Improves microenvironment; synergistically enhances inherent therapeutic effects of BMSCs [11]. | Improved outcomes in rat IRI model when administered (5 mg/kg/day) before and after MSC transplantation [11]. |
| Iron-Quercetin Complex (IronQ) [71] | Metal-flavonoid complex | Upregulates expression and secretion of HGF in MSCs; activates HGF/c-Met pathway to suppress tubular cell apoptosis [71]. | Significantly improved renal function and reduced tubular cell apoptosis in cisplatin-induced AKI mice compared to MSC-only therapy [71]. |
| Serelaxin (RLX) [70] | Recombinant human relaxin-2; anti-fibrotic | Reduces fibrotic renal environment, improving viability and functionality of transplanted BM-MSCs [70]. | Enhanced anti-fibrotic efficacy and renoprotection in normotensive and hypertensive preclinical CKD models [70]. |
Detailed Experimental Protocols
Protocol 1: Chlorzoxazone (CZ) Preconditioning of MSCs
This protocol details the process of preconditioning human Umbilical Cord MSCs (hUCMSCs) with Chlorzoxazone to enhance their immunomodulatory potency for AKI treatment [11].
Reagents and Materials
- Human Umbilical Cord Mesenchymal Stem Cells (hUCMSCs)
- Chlorzoxazone (CZ) (e.g., Sigma-Aldrich, C4390)
- Standard MSC culture medium (e.g., DMEM/F12 supplemented with 10% FBS and 1% Penicillin-Streptomycin)
- Phosphate Buffered Saline (PBS)
- Dimethyl Sulfoxide (DMSO)
Preconditioning Procedure
- CZ Solution Preparation: Prepare a concentrated stock solution of CZ in DMSO. Subsequently, dilute the stock solution in standard MSC culture medium to achieve a final working concentration. The final concentration of DMSO in the culture medium should not exceed 0.1% (v/v).
- Cell Seeding: Seed hUCMSCs at an appropriate density (e.g., 5,000 - 8,000 cells/cm²) in standard culture medium and allow them to adhere overnight.
- Preconditioning Incubation: After cell adherence, carefully aspirate the standard medium and replace it with the CZ-containing medium. Incubate the cells for a predetermined period (e.g., 24-48 hours) at 37°C with 5% CO₂.
- Cell Harvesting: Following the incubation period, wash the preconditioned MSCs with PBS to remove residual CZ. Gently detach the cells using a standard method like trypsinization.
- Transplantation: Resuspend the harvested CZ-preconditioned MSCs in a sterile saline solution for immediate transplantation into the AKI model.
Application In Vivo
- AKI Model: Thy1.1 antibody-induced AKI model in rats [11].
- Administration: Administer CZ-preconditioned hUCMSCs via a suitable route (e.g., intravenous or renal subcapsular injection).
- Assessment: Evaluate efficacy by monitoring renal function (e.g., serum creatinine, BUN) and histological analysis of kidney tissues for inflammation and necrosis.
Protocol 2: Iron-Quercetin (IronQ) Preconditioning of MSCs
This protocol uses an Iron-Quercetin complex to pre-treat MSCs, boosting their secretion of Hepatocyte Growth Factor (HGF) to enhance anti-apoptotic effects in AKI [71].
Reagents and Materials
- Mesenchymal Stem Cells (MSCs)
- Iron-Quercetin complex (IronQ)
- Standard MSC culture medium
- PBS
- HGF Neutralizing Antibody (for mechanistic validation)
- c-Met inhibitor (e.g., PHA-665752, for mechanistic validation)
Preconditioning and Mechanistic Workflow
The following diagram illustrates the key steps of the IronQ preconditioning protocol and the subsequent mechanistic validation experiments.
Preconditioning Procedure
- IronQ Preparation: Prepare a stock solution of the Iron-Quercetin complex as per synthetic protocol.
- MSC Preconditioning: Culture MSCs in standard medium supplemented with the IronQ complex for the determined optimal period.
- In Vivo Transplantation: Induce AKI in male C57BL/6 mice using cisplatin. Intravenously administer 1×10⁶ IronQ-preconditioned MSCs (MSCIronQ) [71].
- Efficacy Assessment: Analyze renal function (serum creatinine, BUN) and kidney histology (tubular cell apoptosis via TUNEL staining) 3 days post-treatment.
Mechanistic Validation
- In Vitro Apoptosis Assay: Induce apoptosis in mouse tubular epithelial cells (mTECs) with cisplatin. Treat injured mTECs with conditioned medium from MSCIronQ.
- Pathway Inhibition: To confirm the HGF/c-Met pathway's role, include control groups where the conditioned medium is pre-incubated with an HGF-neutralizing antibody or where mTECs are treated with a c-Met-specific pharmacological inhibitor.
- Outcome Measurement: Evaluate apoptosis levels using TUNEL assay, Western blotting for apoptotic markers, and RT-PCR.
Protocol 3: Ultrasound-Guided Renal Subcapsular Transplantation with Pharmacological Support
This protocol combines a novel minimally invasive delivery method for MSCs with adjunct pharmacological therapy to maximize cell retention and efficacy [72].
Reagents and Materials
- Bama minipigs (15–20 kg)
- MSCs (P6-P8 passages)
- Cisplatin (for AKI induction)
- Anesthetics (Xylazine hydrochloride, Zoletil 50)
- Ultrasound system (e.g., Mindray M9) with linear probe
- Disposable needle (0.7 × 80 mm)
- Central venous catheter set (e.g., ARROW ES-04301)
- Potassium chloride (for euthanasia)
Transplantation Procedure
- AKI Model Establishment: Induce AKI in minipigs by intravenous injection of cisplatin (3.8 mg/kg) combined with a hydration regimen (4% of body weight pre-hydration and 2% post-hydration) to ensure animal survival and mimic clinical AKI [72].
- Ultrasound-Guided Catheterization:
- Anesthetize the minipig and position it for ultrasound access to the kidney.
- Using ultrasound guidance, identify the inferior pole of the left kidney and select a puncture site.
- Make a small (3 mm) incision at the site. Under continuous ultrasound guidance, inject saline via a needle to create a space between the renal tissue and the capsule.
- Insert the central venous catheter into this subcapsular space.
- Cell Administration:
- For single transplantation: Inject MSCs (2 × 10⁶ cells/kg) in 5 mL saline via the catheter at 6 hours post-cisplatin injection.
- For multiple transplantation: Inject a total of 4 × 10⁶ cells/kg in divided doses (e.g., 2 × 10⁶ cells/kg at 6 hours, followed by 1 × 10⁶ cells/kg at 2 days and 4 days post-cisplatin) [72].
- Adjunct Pharmacological Therapy: Administer supportive or synergistic drugs (e.g., statins based on Protocol 1) as required by the experimental design.
- Monitoring and Analysis: Monitor survival and renal function (SCr, BUN). Collect kidney tissue samples via ultrasound-guided percutaneous puncture or post-euthanasia for histology (PAS, Masson, TUNEL staining) and molecular analysis.
The Scientist's Toolkit: Key Research Reagents
Table 2: Essential Research Reagents for MSC-Pharmacology Combination Studies
| Reagent / Material | Function / Application | Examples / Notes |
|---|---|---|
| Chlorzoxazone (CZ) [11] | Preconditioning agent to enhance MSC immunomodulation. | FDA-approved drug; use in vitro for MSC preconditioning before transplantation. |
| Iron-Quercetin Complex (IronQ) [71] | Preconditioning agent to boost HGF secretion from MSCs. | Synthetic complex; specific formulation and concentration require optimization. |
| Serelaxin (RLX) [70] | Anti-fibrotic agent co-administered to improve MSC microenvironment. | Recombinant human relaxin-2; administer in conjunction with MSC therapy. |
| HGF Neutralizing Antibody [71] | Tool for mechanistic validation of HGF/c-Met pathway involvement. | Used in vitro to block the function of HGF in conditioned medium assays. |
| c-Met Inhibitor [71] | Pharmacological blocker of HGF receptor for pathway validation. | e.g., PHA-665752; can be used in vitro and in vivo. |
| Central Venous Catheter Set [72] | Enables minimally invasive renal subcapsular delivery of MSCs. | e.g., ARROW ES-04301; critical for large animal model studies. |
| High-Resolution Ultrasound [72] | Guides precise catheter placement for renal subcapsular injection. | e.g., Mindray M9 system; essential for survival surgeries in large animals. |
Combining MSC-based therapy with pharmacological agents represents a transformative strategy for overcoming the significant clinical challenges in treating AKI. The protocols detailed herein—ranging from simple MSC preconditioning with agents like Chlorzoxazone and Iron-Quercetin to sophisticated delivery techniques like ultrasound-guided renal subcapsular transplantation—provide a robust experimental framework for researchers. The synergistic effects achieved through these combinations enhance MSC survival, amplify their paracrine functions, and directly mitigate pathological processes, leading to significantly improved renal outcomes in preclinical models. By leveraging these approaches, drug development professionals and translational scientists can accelerate the development of more effective, multi-targeted therapies for acute kidney injury.
The selection of an optimal mesenchymal stem cell (MSC) source is a critical determinant for the success of renal cortex injection-based therapies for acute kidney injury (AKI). While MSCs from various sources share core characteristics—multilineage differentiation potential, self-renewal capacity, and paracrine activity—they exhibit significant differences in their secretory profiles, expansion capabilities, and in vivo functional properties that directly influence therapeutic outcomes [11] [42] [1]. These differences arise from their distinct embryological origins and tissue-specific niches, resulting in variations in cytokine secretion, growth factor production, immunomodulatory potential, and homing capabilities to injured renal tissues [42] [2]. This document provides a structured comparison of four prominent MSC sources—bone marrow (BM-MSCs), adipose tissue (AD-MSCs), umbilical cord (UC-MSCs), and tonsil tissue (T-MSCs)—to guide researchers in selecting the most appropriate cell source for AKI therapeutic development based on scientific evidence and practical experimental considerations.
Table 1: Key Functional Characteristics of Different MSC Sources in AKI Models
| MSC Source | Proliferation Capacity | Key Paracrine Factors | Documented Mechanisms in AKI | Therapeutic Efficacy Evidence |
|---|---|---|---|---|
| Bone Marrow (BM-MSC) | Moderate [3] | HGF, VEGF, FGF, IGF-1 [11] [42] | Immunomodulation, anti-apoptosis, angiogenesis [11] [1] | Improved function in IRI & cisplatin models [11] [42] |
| Adipose Tissue (AD-MSC) | High [73] | VEGF, FGF, EGF, PGF [73] | Anti-oxidative stress, anti-inflammatory, mitochondrial transfer [11] [12] | Dose-dependent renal repair via EVs in cisplatin-AKI [12] |
| Umbilical Cord (UC-MSC) | High [74] [42] | HGF, VEGF, SDF-1, integrins [11] | Inflammation reduction (↓TNF-α, ↓IL-6, ↑IL-10) [74] | Reduced damage in IRI models [74] |
| Tonsil (T-MSC) | Very High [3] [75] | Anti-oxidants (GPx, catalase) [3] [75] | Anti-apoptosis, anti-oxidative, ER stress amelioration [3] [75] | Incorporated into tubules; reduced BUN/Cr in GM-AKI [3] [75] |
Table 2: Comparative Analysis of MSC Sources for Practical Research Applications
| Parameter | Bone Marrow (BM-MSC) | Adipose Tissue (AD-MSC) | Umbilical Cord (UC-MSC) | Tonsil (T-MSC) |
|---|---|---|---|---|
| Tissue Availability | Invasive harvest; limited donor supply [3] | Minimally invasive; abundant tissue [73] | Non-invasive; perinatal waste tissue [74] [42] | Surgical discard tissue; easy access [3] [75] |
| Expansion Potential | Moderate; senesces with passages [2] | High; maintains viability [73] | High; primitive cell properties [42] | Highest reported yield; fast proliferation [3] [75] |
| Immunogenicity | Low (allogeneic possible) [1] | Low (allogeneic possible) [42] | Low (allogeneic possible) [42] | Low (allogeneic possible) [3] |
| Clinical Trial Evidence | Extensive human trials [42] | Emerging human trials [42] [73] | Multiple clinical trials [42] | Preclinical stage (animal models) [3] [75] |
Experimental Protocols for MSC Evaluation in AKI Models
Objective: To establish standardized protocols for isolating and expanding MSCs from BM, adipose, umbilical cord, and tonsillar tissues for renal cortex injection studies.
Materials:
- Digestion Solution: Collagenase Type I (210 U/mL for tonsil; [3]), DNase (10μg/mL; [3])
- Culture Medium: High-glucose DMEM or DMEM/F12 [3] [12] supplemented with 10% FBS [3] [12] and 1% penicillin/streptomycin [3] [12]
- Expansion Surface: T75 or T175 culture flasks [3]
- Passaging Reagent: 0.25% trypsin [12]
Methodology:
- Tonsil-MSC Isolation (T-MSCs):
- Obtain human tonsils from tonsillectomy procedures with appropriate ethical approval and informed consent [3].
- Mince tissue thoroughly and digest in medium containing collagenase type I (210 U/mL) and DNase (10μg/mL) [3].
- Filter through a cell strainer to obtain single-cell suspension [3].
- Culture in high-glucose DMEM with 10% FBS and 1% penicillin/streptomycin at 37°C with 5% CO₂ [3].
Adipose-MSC Isolation (AD-MSCs):
- Collect adipose tissue (e.g., from inguinal fat pads in rabbits) [73].
- Wash with PBS thoroughly and mince into small pieces [73].
- Digest with collagenase solution (37°C for 30 minutes) [73].
- Centrifuge to separate stromal vascular fraction containing MSCs [73].
- Culture in DMEM/F12 medium with 10% FBS and 1% penicillin/streptomycin [12].
Bone Marrow-MSC Isolation (BM-MSCs):
Umbilical Cord-MSC Isolation (UC-MSCs):
Quality Control:
- Use cells between passages 3-8 for experiments to ensure optimal functionality [12].
- Characterize MSCs by flow cytometry for positive (CD73, CD90, CD105) and negative (CD34, CD45) markers [3].
- Verify multilineage differentiation potential into adipogenic, osteogenic, and chondrogenic lineages [3].
Protocol: Renal Cortex Injection in AKI Animal Models
Objective: To deliver MSCs directly into the renal cortex of AKI models for evaluating paracrine-mediated repair mechanisms.
Materials:
- Animal Model: Sprague-Dawley rats (200-250g) [3] or Nude mice (8-10 weeks) [12]
- AKI Induction Agents: Gentamicin (70 mg/kg/day IP for 10 days; [3]), cisplatin (single IP injection; [12]), or ischemia-reperfusion injury (renal artery clamping; [74])
- Surgical Instruments: Microsurgical tools, hemostatic forceps [73]
- Injection System: Hamilton syringe (50-100μL capacity), 30G needle [2]
- Cell Preparation: MSCs (1×10⁷ cells/mL in saline; [3]) labeled with PKH26 fluorescent dye [3] or GFP [1] for tracking
Methodology:
- AKI Model Establishment:
- For gentamicin-induced AKI: Administer gentamicin (70 mg/kg/day) via intraperitoneal injection for 10 days [3].
- For cisplatin-induced AKI: Administer single dose of cisplatin via intraperitoneal injection [12].
- For ischemia-reperfusion injury: Anesthetize animal, make flank incision to expose kidney, clamp renal artery for 45 minutes [73], then release clamp for reperfusion.
Renal Cortex Injection Procedure:
- Anesthetize animal and secure in lateral position [2].
- Make small flank incision (1-2cm) to expose the kidney [73] [2].
- Load prepared MSCs (1×10⁷ cells in 500μL for rats; [3]) into Hamilton syringe with 30G needle.
- Under direct visualization, insert needle superficially into renal cortex at multiple sites (2-3 sites per kidney) [2].
- Inject cells slowly (10-20μL per site) to minimize reflux and tissue damage [2].
- Apply gentle pressure with sterile cotton swab after needle withdrawal to prevent leakage [2].
- Return kidney to anatomical position and suture muscle and skin layers [73].
Post-operative Monitoring:
- Monitor animals until fully recovered from anesthesia.
- Administer analgesics as approved by animal ethics committee.
- Track renal function via serum creatinine and BUN measurements at days 1, 3, 7, and 14 post-injection [3].
Validation Steps:
- Confirm MSC engraftment using fluorescence microscopy for PKH26-labeled cells [3] or immunohistochemistry for human nuclear antigen [3].
- Assess tubular damage using periodic acid-Schiff staining and semiquantitative scoring (0-4 scale) [3].
- Evaluate apoptosis via TUNEL staining [3] and oxidative stress via urinary 8-OHdG measurement [3].
Signaling Pathways in MSC-Mediated Renal Repair
The therapeutic effects of MSCs in AKI are primarily mediated through paracrine modulation of key signaling pathways that promote renal repair, reduce inflammation, and mitigate cellular stress.
MSC Signaling Pathways in AKI Recovery
The diagram illustrates the key molecular mechanisms through which MSCs from different sources mediate renal repair. Specific pathways are particularly prominent depending on MSC source:
- T-MSCs demonstrate robust anti-apoptotic and anti-oxidative effects, significantly decreasing Bax, cytochrome c, and cleaved caspase while increasing Bcl-2 expression [3]. They also enhance expression of antioxidant enzymes GPx and catalase [3].
- UC-MSCs strongly modulate inflammatory responses, reducing pro-inflammatory cytokines TNF-α and IL-6 while increasing anti-inflammatory IL-10 [74].
- AD-MSC-EVs specifically target CX3CR1+ macrophages and promote their polarization toward reparative M2 phenotype through suppression of the TXNIP-IKKα/NF-κB signaling pathway [12].
- BM-MSCs show balanced activity across multiple pathways, with documented paracrine secretion of HGF, VEGF, and other growth factors that promote angiogenesis and tissue repair [11] [42].
The Scientist's Toolkit: Essential Research Reagent Solutions
Table 3: Key Research Reagents for MSC-based AKI Studies
| Reagent/Category | Specific Examples | Research Function | Application Notes |
|---|---|---|---|
| Cell Culture Media | High-glucose DMEM, DMEM/F12 [3] [12] | MSC expansion and maintenance | Supplement with 10% FBS [3] [12] and 1% penicillin/streptomycin [3] [12] |
| Characterization Antibodies | CD73, CD90, CD105 (positive) [3]; CD34, CD45 (negative) [3] | MSC phenotype confirmation | Flow cytometry verification essential for publication-quality studies |
| Cell Tracking Dyes | PKH26 [3], DiI [12], GFP labeling [1] | In vivo cell localization | PKH26 provides stable membrane labeling for renal cortex engraftment studies [3] |
| AKI Induction Agents | Gentamicin [3], cisplatin [12], ischemia-reperfusion [74] | Disease modeling | Gentamicin: 70 mg/kg/day IP for 10 days [3]; Cisplatin: single IP injection [12] |
| Renal Function Assays | Creatinine assay kit [3], BUN assay kit [3] | Therapeutic efficacy assessment | Commercial kits available (e.g., QuantiChrom) [3] |
| Histology Reagents | Periodic acid-Schiff stain [3], TUNEL assay [3] | Tissue damage and apoptosis assessment | Semiquantitative scoring (0-4) for tubular damage [3] |
| Oxidative Stress Markers | 8-OHdG ELISA [3], antioxidant enzyme antibodies (GPx, catalase) [3] | Oxidative damage evaluation | Urinary 8-OHdG measurement correlates with oxidative stress [3] |
| Cytokine Analysis | TNF-α, IL-6, IL-10 primers [74] or ELISA | Inflammation monitoring | RT-PCR or multiplex ELISA for quantification |
The selection of an optimal MSC source for renal cortex injection in AKI depends on the specific research objectives and mechanistic focus. BM-MSCs represent the most extensively characterized source with substantial clinical trial data [42]. UC-MSCs offer high proliferation capacity and potent immunomodulatory effects [74] [42]. AD-MSCs provide readily accessible tissue and strong paracrine activity, particularly through their extracellular vesicles [12] [73]. T-MSCs demonstrate exceptional proliferation potential and robust integration into damaged renal tubules with significant anti-apoptotic and anti-oxidative effects [3] [75].
For researchers prioritizing clinical translation, BM-MSCs and UC-MSCs have the strongest human trial evidence [42]. For mechanistic studies focusing on oxidative stress and cellular integration, T-MSCs offer distinct advantages [3] [75]. For paracrine-focused therapies without cellular integration, AD-MSCs and their extracellular vesicles present promising cell-free alternatives [12]. The renal cortex injection route directly addresses the challenge of poor cellular retention observed with systemic administration, potentially amplifying the therapeutic benefits of all MSC sources [2].
Efficacy Assessment and Clinical Translation Prospects
The therapeutic efficacy of mesenchymal stem cells (MSCs) in acute kidney injury (AKI) is consistently demonstrated in preclinical models through significant improvements in key renal function biomarkers. The following tables summarize quantitative data on serum creatinine (SCr) and blood urea nitrogen (BUN) improvements from recent studies.
Table 1: Functional Improvements with Preconditioned MSCs in Rodent AKI Models
| Preconditioning Strategy | AKI Model | MSC Source | SCr Reduction | BUN Reduction | Reference |
|---|---|---|---|---|---|
| Hypoxia (1% O₂) | Ischemia-Reperfusion Injury (IRI) | Human Adipose | Significant restoration | Significant restoration | [11] |
| Hypoxia (5% O₂) | Gentamicin-induced | Human Umbilical Cord | Marked amelioration | Marked amelioration | [11] |
| Hypoxia (CoCl₂ mimic) | Ischemia-Reperfusion Injury (IRI) | Rat Bone Marrow | Improved kidney preservation | Improved kidney preservation | [11] |
| Chlorzoxazone | Thy1.1 Antibody-induced | Human Umbilical Cord | Attenuated renal dysfunction | Attenuated renal dysfunction | [11] |
| Atorvastatin | Ischemia-Reperfusion Injury (IRI) | Rat Bone Marrow | Improved function | Improved function | [11] |
Table 2: Functional Improvements with MSC-Derived Products and Alternative MSC Sources
| Cell Type / Product | AKI Model | Species | Key Functional Outcomes | Reference |
|---|---|---|---|---|
| Tonsil-derived MSCs (T-MSCs) | Gentamicin-induced | Rat | ↓ BUN, ↓ SCr, lower tubular damage score | [3] |
| MSC-derived Extracellular Vesicles (EVs) | Cisplatin-induced | In vitro (HK-2 cells) | Enhanced cell proliferation, suppressed apoptosis | [76] |
| MSC-derived Exosomes | Ischemia-Reperfusion Injury (IRI) | Mouse | Reduced plasma creatinine levels, less tubular necrosis | [77] |
| Induced Pluripotent Stem Cell (iPSC)-EVs | Not Specified | In vitro | Mitigated cell death and tissue damage | [77] |
Detailed Experimental Protocols for Preclinical AKI Models
Protocol: Establishing a Gentamicin-Induced AKI Model and Evaluating T-MSCs
This protocol is adapted from a study demonstrating the efficacy of tonsil-derived MSCs [3].
- Animal Model: Male Sprague-Dawley rats (200–250 g).
- AKI Induction:
- Agent: Gentamicin (GM).
- Dosage and Route: 70 mg/kg/day via intraperitoneal injection.
- Duration: 10 consecutive days.
- Cell Preparation and Administration:
- MSC Source: Human tonsil-derived MSCs (T-MSCs).
- Cell Labeling: Label T-MSCs with PKH26 fluorescent dye for in-vivo tracking.
- Dosage: 1 x 10⁷ cells suspended in 500 μL saline.
- Route and Timing: Single intravenous injection via the tail vein on day 11 (one day after the last GM dose).
- Functional Endpoint Analysis (Day 16):
- Blood Collection: Terminal blood collection via cardiac puncture or inferior vena cava under anesthesia.
- Biochemical Assays:
- Serum Creatinine (SCr): Quantified using a commercial QuantiChrom creatinine assay kit.
- Blood Urea Nitrogen (BUN): Quantified using a commercial QuantiChrom urea nitrogen assay kit.
- Histological and Molecular Analysis:
- Kidney Tissue Harvest: Perfuse kidneys with cold PBS, followed by fixation (e.g., Methyl Carnoy's solution) for histology or snap-freezing for molecular analysis.
- Tubular Damage Scoring: Periodic acid-Schiff (PAS) stained sections are graded (0-4) based on the percentage of damaged tubules.
- Apoptosis Assay: TUNEL staining on kidney sections to quantify apoptotic cells.
- Oxidative Stress Measurement: Urinary 8-hydroxy-2'-deoxyguanosine (8-OHdG) measured by ELISA.
Protocol: Hypoxic Preconditioning of MSCs for AKI Therapy
This protocol outlines the general methodology for enhancing MSC efficacy prior to transplantation, as described in multiple studies [11].
- MSC Culture:
- Source MSCs from bone marrow, adipose, or umbilical cord tissue.
- Culture in standard expansion medium (e.g., Dulbecco's Modified Eagle Medium with 10% FBS) at 21% O₂, 5% CO₂.
- Hypoxic Preconditioning:
- Equipment: Use a tri-gas incubator or hypoxia chamber.
- Conditions: Culture MSCs at a defined low oxygen tension (e.g., 1% or 5% O₂) for 24-72 hours prior to harvest.
- Alternative Chemical Mimic: Treat MSCs with Cobalt Chloride (CoCl₂, e.g., 100-200 μM for 24h) to chemically induce hypoxia-mimetic responses.
- Validation of Preconditioning:
- Western Blot: Confirm upregulation of hypoxia-inducible factor 1-alpha (HIF-1α) in preconditioned MSCs.
- ELISA/RT-qPCR: Assess enhanced secretion/expression of target factors (e.g., VEGF, HGF, SDF-1).
- Administration in AKI Models:
- Harvest and resuspend preconditioned MSCs in sterile saline.
- Administer intravenously or via renal cortex injection at a predetermined dose (e.g., 1-5 x 10⁶ cells) shortly after AKI induction (e.g., post-ischemia or during nephrotoxic injury).
Signaling Pathways and Experimental Workflow
MSC Paracrine Signaling in AKI Repair
This diagram illustrates the key paracrine mechanisms by which MSCs and their secreted products mediate functional recovery in AKI, leading to the observed improvements in SCr and BUN.
Preclinical Workflow for MSC Evaluation
This diagram outlines the sequential workflow for evaluating the functional efficacy of MSCs in a preclinical AKI model, from establishment to analysis.
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagent Solutions for MSC-based AKI Research
| Reagent / Kit | Vendor Examples | Function in Protocol |
|---|---|---|
| QuantiChrom Creatinine Assay Kit | BioAssay Systems | Enables accurate, colorimetric quantification of serum creatinine levels as a primary endpoint for renal function. |
| QuantiChrom Urea Assay Kit | BioAssay Systems | Facilitates measurement of Blood Urea Nitrogen (BUN) concentrations in serum samples. |
| PKH26 Red Fluorescent Cell Linker Kit | Sigma-Aldrich | Used for stable, long-term labeling of MSCs to track their migration, homing, and retention in renal tissue post-injection. |
| In Situ Cell Death Detection Kit (TUNEL) | Roche Diagnostics | Allows for the fluorescent labeling of DNA fragmentation in tissue sections, enabling quantification of apoptotic cells in the kidney. |
| 8-OHdG ELISA Kit | Cell Biolabs, Inc. | Provides a quantitative measure of oxidative stress in the kidney by detecting 8-hydroxy-2'-deoxyguanosine in urine or tissue homogenates. |
| Hypoxia Incubator Chamber | STEMCELL Technologies, Billups-Rothenberg | Creates a controlled, low-oxygen environment (e.g., 1-5% O₂) for the preconditioning of MSCs prior to their therapeutic application. |
| Anti-HIF-1α Antibody | Cell Signaling Technology, Abcam | Validates the success of hypoxic preconditioning in MSCs via Western Blot analysis. |
Within research on paracrine therapy for Acute Kidney Injury (AKI) using renal cortex-injected Mesenchymal Stem Cells (MSCs), precise histological assessment is fundamental for evaluating injury severity and documenting regenerative efficacy. This document provides detailed application notes and standardized protocols for scoring tubular injury and identifying key regeneration markers, serving as an essential toolkit for preclinical research and drug development. The systematic quantification of histopathological changes enables robust correlation between structural repair and functional recovery, providing critical evidence for therapeutic efficacy in AKI models.
Semiquantitative Scoring of Tubular Injury
A standardized scoring system for Acute Tubular Injury (ATI) allows for objective histologic grading, which correlates with clinical parameters of AKI severity. The following section outlines a validated semiquantitative approach.
Tubular Injury Scoring System
A widely adopted scoring system, as utilized in recent studies, evaluates three key morphological features on hematoxylin and eosin (H&E) stained sections: tubular epithelial simplification, cell sloughing, and mitosis [78]. Each feature is scored on a scale of 0-3, and a total injury score is calculated.
Table 1: Semiquantitative Scoring System for Acute Tubular Injury
| Histological Feature | Definition | Scoring Criteria | Score |
|---|---|---|---|
| Epithelial Simplification | Tubular cross-sections with flattened epithelial cell cytoplasm, loss of brush border, and luminal dilatation [78] [79]. | 0: 0% of tubules1: <25% of tubules2: 25-50% of tubules3: >50% of tubules | 0-3 |
| Cell Sloughing | Presence of free-floating cells in the tubular lumen without attachment to basement membrane [78]. | 0: No sloughing1: 1 tubule with sloughing2: 2 tubules with sloughing3: ≥3 tubules with sloughing | 0-3 |
| Mitotic Figures | Tubular epithelial cells in any distinct mitotic phase [78]. | 0: No mitosis1: 1 mitosis2: 2 mitoses3: ≥3 mitoses | 0-3 |
| Total Injury Score | Calculation: (2 × Simplification score) + (2 × Mitosis score) + Cell sloughing score [78] | Grading:≤2: Mild3: Equivocal≥4: Severe |
Protocol for Light Microscopy and H&E Staining
Sample Preparation:
- Fixation: Immerse kidney tissue samples in 10% Neutral Buffered Formalin for 24-48 hours [80].
- Processing: Dehydrate the fixed tissue through a graded series of ethanol, clear with xylene, and infiltrate with paraffin wax [80].
- Embedding: Orient tissue in a mold filled with molten paraffin and allow to solidify [80].
- Sectioning: Cut 3-5 μm thick sections using a microtome and float onto charged glass slides [80] [3].
H&E Staining Protocol (Manual Regressive Staining): This protocol follows a standard regressive method, which involves over-staining and then differentiating to achieve optimal contrast [81].
Table 2: H&E Staining Protocol for Renal Histology
| Step | Reagent | Duration | Purpose |
|---|---|---|---|
| 1 | Xylene | 2 minutes | Dewaxing |
| 2 | Xylene | 2 minutes | Complete dewaxing |
| 3 | 100% Ethanol | 2 minutes | Dehydration |
| 4 | 100% Ethanol | 2 minutes | Complete dehydration |
| 5 | 95% Ethanol | 2 minutes | Hydration |
| 6 | Running Water | 2 minutes | Complete hydration |
| 7 | Hematoxylin (e.g., Harris) | 3 minutes | Nuclear staining |
| 8 | Running Water | 1 minute | Rinse |
| 9 | Acid Differentiation (e.g., 1% HCl) | 1 minute | Remove excess hematoxylin |
| 10 | Running Water | 1 minute | Rinse |
| 11 | Bluing Solution (e.g., Scott's) | 1 minute | Convert stain to blue |
| 12 | Running Water | 1 minute | Rinse |
| 13 | 95% Ethanol | 1 minute | Dehydration |
| 14 | Eosin Y | 45 seconds | Cytoplasmic staining |
| 15 | 95% Ethanol | 1 minute | Dehydration & differentiation |
| 16 | 100% Ethanol | 1 minute | Dehydration |
| 17 | 100% Ethanol | 1 minute | Complete dehydration |
| 18 | Xylene | 2 minutes | Clearing |
| 19 | Xylene | 2 minutes | Complete clearing |
| 20 | Coverslipping | - | Mounting with resinous medium |
Correlation with Clinical Parameters and Limitations
Application of this scoring system has demonstrated a significant positive correlation between total histologic scores and serum creatinine levels at presentation, confirming its relevance for assessing AKI severity [78]. However, it is critical to note that while these scores indicate injury severity, studies have shown that they alone may not predict clinical recovery patterns. Factors such as patient age >60 years, hypertension, diabetes, and a high chronicity score on biopsy (reflecting interstitial fibrosis and tubular atrophy) are more reliable indicators of delayed or partial recovery [78].
Quantitative Analysis and Digital Pathology
Beyond semiquantitative scoring, morphometric analysis provides robust, unbiased quantitative data on histopathological changes.
Protocol for Morphometric Analysis of Tubular Injury
This protocol utilizes image analysis software (e.g., Image-Pro Plus, Definiens Tissue Studio) to quantify features from H&E-stained whole-slide images [82].
- Image Acquisition: Scan H&E-stained sections using a virtual slide scanner at 20x or 40x magnification to generate high-resolution whole-slide images.
- Region of Interest (ROI) Definition: Manually delineate the cortical area for analysis, excluding medulla and large vessels.
- Nuclear Detection: Use software to detect tubular epithelial nuclei based on hematoxylin staining intensity, size, and shape. Set thresholds to exclude nuclei from infiltrating cells and glomeruli.
- Simulated Cell Delineation: In areas of simplification, the software can expand the region from the detected nucleus to simulate the cytoplasmic boundary of tubular cells, allowing for measurement of simulated cell area as an indicator of hypertrophy and simplification [82].
- Vacuole and Cast Measurement: To quantify vacuolation or specific casts, set detection parameters based on area and roundness to identify and measure unstained circular objects within the tubular epithelium or lumen, respectively [82].
- Infiltrating Cell Quantification: Identify mononuclear inflammatory cells based on nuclear size, intensity, and morphology. Differentiate from tubular nuclei by setting a smaller size range (e.g., 5-45 μm²) and using "fill holes" functions to ensure each nucleus is counted as a single object [82].
Limitations: This method may struggle with severely clustered infiltrating cells and requires careful validation to distinguish different cell types. Vacuoles in the kidney can be challenging to quantify specifically due to the presence of other unstained regions like tubular lumina [82].
Immunohistochemical Markers of Injury and Repair
Immunohistochemistry (IHC) provides critical insights into cellular responses, immune activity, and regenerative processes.
Macrophage Phenotyping in AKI
Macrophages play a dual role in AKI, with M1 (pro-inflammatory) and M2 (pro-repair) phenotypes influencing injury and recovery [78].
Protocol for Macrophage Immunohistochemistry:
- Section Preparation: Cut 3-5 μm sections from formalin-fixed, paraffin-embedded (FFPE) tissue onto charged slides.
- Deparaffinization and Rehydration: Follow steps 1-6 of the H&E protocol.
- Antigen Retrieval: Perform heat-induced epitope retrieval using a citrate-based or EDTA-based buffer (pH 6.0 or 9.0) in a pressure cooker or water bath [80].
- Endogenous Peroxidase Blocking: Incubate sections with 3% hydrogen peroxide for 10-15 minutes.
- Protein Block: Apply a normal serum block from the secondary antibody species for 30 minutes.
- Primary Antibody Incubation: Apply antibodies against pan-macrophage and phenotype-specific markers. Incubate according to manufacturer's instructions (typically overnight at 4°C).
- Secondary Antibody and Detection: Apply a species-specific biotinylated secondary antibody, followed by an avidin-biotin-enzyme complex (ABC). Visualize with 3,3'-Diaminobenzidine (DAB) to produce a brown precipitate.
- Counterstaining and Mounting: Counterstain lightly with hematoxylin, dehydrate, clear, and mount.
Quantification: Count positively stained interstitial cells across the entire cortical area. Calculate cell density by dividing the cell count by the exact cortical area measured using digital image analysis software [78]. Studies show that densities of CD68+ and CD163+ macrophages correlate with serum creatinine levels, reflecting injury severity [78].
Markers of Tubular Regeneration and Stress
Table 3: Key Immunohistochemical Markers for AKI Research
| Marker | Cellular Localization | Function and Significance | Application in MSC Therapy Studies |
|---|---|---|---|
| Kim-1 | Tubular Epithelium | Transmembrane glycoprotein upregulated in damaged proximal tubules; a sensitive marker of injury [3]. | Documents the reduction of tubular injury following MSC administration. |
| NGAL | Tubular Epithelium | Secreted protein involved in iron trafficking; rapidly elevated in urine and tissue after AKI [3]. | Indicates attenuation of early tubular damage by MSC paracrine effects. |
| Bax / Bcl-2 | Tubular Epithelium (Cytoplasm) | Bax (pro-apoptotic) and Bcl-2 (anti-apoptotic) proteins regulate apoptosis. A high Bax/Bcl-2 ratio indicates apoptotic activity [3]. | Demonstrates the anti-apoptotic effect of MSCs (reduced Bax/Bcl-2 ratio). |
| Cleaved Caspase-3 | Tubular Epithelium (Nucleus/Cytoplasm) | Activated executioner caspase in the apoptosis pathway [3]. | Directly shows suppression of apoptotic cell death after MSC treatment. |
| 8-OHdG | Tubular Epithelium (Nucleus) | Marker of oxidative DNA damage [3]. | Used to validate the anti-oxidative properties of MSCs (reduced staining). |
The Scientist's Toolkit: Research Reagent Solutions
Table 4: Essential Reagents for Tubular Injury and Regeneration Studies
| Reagent / Assay | Vendor Examples | Function in Protocol |
|---|---|---|
| Primary Antibodies (Anti-CD68, CD163, HLA-DR, Kim-1, NGAL) | Abcam, Cell Signaling Technology, Dako | Target-specific detection of macrophages, injury, and stress markers via IHC. |
| IHC Detection Kit (e.g., ABC, HRP Polymer) | Vector Laboratories, Agilent Dako | Amplifies signal from primary antibody for visualization with chromogens like DAB. |
| Hematoxylin & Eosin Staining Kits | Sigma-Aldrich, Leica Biosystems | Provides standardized reagents for consistent routine histology. |
| TUNEL Assay Kit | Roche Diagnostics | Fluorescent or colorimetric detection of DNA fragmentation in apoptotic cells. |
| Digital Slide Scanner | Leica Biosystems, Philips, Hamamatsu | Creates high-resolution whole-slide images for quantitative analysis. |
| Image Analysis Software | Definiens Tissue Studio, Image-Pro Plus, Visiopharm | Enables automated, quantitative morphometry of histology and IHC. |
| ELISA Kits (e.g., for 8-OHdG) | Cell Biolabs | Quantifies oxidative stress biomarkers in urine or tissue homogenates. |
Signaling Pathways and Experimental Workflow
The following diagrams illustrate the key pathological processes in AKI and the integrated experimental workflow for histological evaluation within an MSC therapy study.
Diagram 1: Key Pathological Signaling in AKI. This diagram outlines the core cellular events following an acute kidney insult, leading to the characteristic histological patterns of Acute Tubular Injury (ATI).
Diagram 2: Histological Workflow for MSC Therapy Evaluation. This workflow integrates routine histology, specialized staining, and digital analysis to comprehensively assess the impact of MSC therapy on kidney injury and repair.
Application Notes and Protocols
Acute kidney injury (AKI) is a prevalent clinical syndrome characterized by rapid renal dysfunction, affecting approximately 13.3 million individuals globally annually [11] [39]. Mesenchymal stem cells (MSCs) have emerged as a promising therapeutic strategy for AKI due to their paracrine capabilities, including immunomodulation, anti-apoptotic effects, and promotion of tissue repair [11] [2]. However, the efficacy of MSC-based therapies is significantly influenced by the delivery route, which impacts cell retention, survival, and engraftment in the kidney [39] [2] [38]. This document provides a comparative analysis of three primary delivery routes—intravenous (IV), intra-arterial (IA), and renal cortex injection—within the context of optimizing paracrine therapy for AKI. Quantitative data, experimental protocols, and reagent solutions are summarized to guide preclinical research.
Quantitative Comparison of Delivery Routes
The table below synthesizes key quantitative findings from preclinical studies comparing IV, IA, and renal cortex injection routes.
Table 1: Comparative Efficacy of MSC Delivery Routes in Preclinical AKI Models
| Delivery Route | Cell Retention & Survival | Functional Outcomes | Histological & Paracrine Benefits | Key Limitations |
|---|---|---|---|---|
| Intravenous (IV) | Low renal retention due to pulmonary first-pass effect; <10% of injected cells reach the kidney [2] [38]. | Limited or inconsistent improvement in serum creatinine (SCr) and glomerular filtration rate (GFR) in clinical trials [39] [44]. | Reduces tubular injury and inflammation in animal models; effects mediated via systemic paracrine signaling [39] [2]. | High cell entrapment in lungs/liver; requires higher doses, increasing risks of embolism and thrombotic complications [2] [38]. |
| Intra-arterial (IA) | Higher renal retention (10–15%) compared to IV [83]; bypasses pulmonary circulation [2] [38]. | Superior to IV in improving SCr and GFR in meta-analyses [39]. | Enhances angiogenesis and reduces fibrosis via localized paracrine action [11] [2]. | Invasive; risk of vascular occlusion and procedural injury [2] [38]. |
| Renal Cortex Injection | Highest retention: Cells retained for ≥14 days using biomaterials (e.g., collagen, hydrogels) [84]. | Significantly reduces SCr and urinary NGAL; promotes long-term functional recovery [83] [84]. | Markedly reduces tubular injury, fibrosis, and apoptosis; amplifies paracrine effects (e.g., HGF secretion) [84] [71]. | Technically challenging; requires surgery; potential for localized injury [84]. |
Key Insights:
- Ranking by Efficacy: Renal cortex injection > IA > IV, based on cell retention and functional/histological outcomes [39] [84].
- Paracrine Enhancement: Local delivery (renal cortex/IA) increases secretion of therapeutic factors (e.g., HGF, VEGF), suppressing apoptosis and inflammation [11] [71].
Experimental Protocols for Delivery Routes
Below are standardized protocols for implementing each delivery route in murine AKI models, derived from cited studies.
Intravenous (IV) Delivery Protocol
- Animal Model: Mice/rats with ischemia-reperfusion injury (IRI) or cisplatin-induced AKI [39] [71].
- Cell Preparation: Suspend 1–2 × 10^6 MSCs in 100–200 µL saline or PBS [39].
- Procedure:
- Inject cells via tail vein using a 29–30G needle.
- Monitor for acute adverse effects (e.g., respiratory distress).
- Validation: Track cells via bioluminescence imaging (BLI) or fluorescence; expect <10% renal retention [38].
Intra-arterial (IA) Delivery Protocol
- Animal Model: Unilateral or bilateral IRI models [38].
- Cell Preparation: Suspend 1–2 × 10^6 MSCs in 100 µL of 0.6% w/v alginate solution [38].
- Procedure:
- Anesthetize and position animal laterally.
- Use ultrasound guidance (e.g., Vevo 2100) with color Doppler to locate the renal artery.
- Insert a 30–33G needle percutaneously into the renal artery under real-time imaging.
- Inject cells slowly (over 30–60 seconds) to avoid reflux.
- Validation: Confirm success via Doppler flow resumption; track cells with BLI/photoacoustic imaging [38].
Renal Cortex Injection Protocol
- Animal Model: Unilateral IRI with contralateral nephrectomy [84].
- Cell Preparation: Encapsulate 2 × 10^6 MSCs in 80 µL collagen I matrix (3 mg/mL, pH 7.4) [84].
- Procedure:
- Expose the kidney via flank incision.
- Subcapsular Injection: Insert needle tangentially under the capsule; inject to create a visible bulge.
- Parenchymal Injection: Inject into multiple cortical sites (e.g., 2–3 deposits).
- Hold needle in place for 15 seconds post-injection to prevent leakage.
- Validation: Histology (CD73 staining) and in vivo imaging (e.g., IVIS) to confirm retention [84].
Signaling Pathways in MSC Paracrine Therapy
Renal cortex delivery enhances paracrine signaling by prolonging MSC survival. The diagram below illustrates key pathways amplified by local MSC engraftment.
Title: MSC Paracrine Signaling in AKI
Mechanistic Insights:
- HGF/c-Met Pathway: Iron-Quercetin preconditioning upregulates HGF in MSCs, activating c-Met and Akt to suppress apoptosis via Bcl-2 [71].
- Anti-inflammatory Effects: Local MSC delivery enhances IL-10 secretion, reducing TNF-α and IL-6 [44].
- Angiogenesis: VEGF secretion promotes vascular repair, particularly in ischemic AKI [11].
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Reagents for MSC Delivery Studies
| Reagent/Material | Function | Example Application |
|---|---|---|
| Collagen I Matrix | Encapsulates MSCs for renal cortex injection, enhancing retention and stability. | Used in subcapsular/parenchymal delivery at 3 mg/mL (pH 7.4) [84]. |
| Hyaluronic Acid (HA) Hydrogel | Shear-thinning scaffold for subcapsular MSC delivery; improves viability post-injection. | Injected under the renal capsule in IRI models [83]. |
| Alginate Solution (0.6% w/v) | Vehicle for IA injection; reduces cell washback during ultrasound-guided delivery. | Renal artery injections in mice [38]. |
| Gold Nanorods (GNRs) | PA imaging contrast agents for tracking MSC localization and retention. | Label MSCs pre-injection; monitor via PA imaging [38]. |
| Iron-Quercetin (IronQ) | Preconditioning agent to enhance HGF secretion and anti-apoptotic effects. | Incubate with MSCs pre-transplantation [71]. |
| CD73 Antibody | Histological validation of human MSC engraftment in kidney tissues. | Immunostaining of tissue sections post-injection [84]. |
Renal cortex injection of MSCs, particularly when combined with biomaterial encapsulation, offers superior efficacy for AKI paracrine therapy compared to IV and IA routes. This approach maximizes cell retention and paracrine factor secretion (e.g., HGF, VEGF), leading to significant functional and histological improvements. Future research should focus on standardizing minimally invasive techniques and optimizing preconditioning strategies to accelerate clinical translation.
Within the advancing field of mesenchymal stem cell (MSC) therapy for acute kidney injury (AKI), the route of cell administration is a critical determinant of both therapeutic efficacy and safety. The potential of renal paracrine therapy hinges on the successful and secure delivery of MSCs to the injured tissue. Intravascular infusion, a common delivery method, carries inherent risks, including pulmonary embolism and renal microvascular occlusion, which can arise from cell aggregation and first-pass lung entrapment. This application note provides a detailed safety profile, synthesizing quantitative data and procedural protocols to assess the risks of embolism and procedure-related injury, thereby supporting the development of safer renal cortex-directed MSC therapies.
Quantitative Safety and Efficacy Profile of MSC Delivery Routes
The choice of administration pathway significantly impacts cell engraftment, retention, and the risk of adverse events. The table below summarizes key findings from preclinical studies comparing different delivery routes for MSC therapy in kidney injury models.
Table 1: Comparative Safety and Efficacy of MSC Administration Routes in Preclinical Models
| Administration Route | Model | Key Findings on Safety & Engraftment | Evidence of Efficacy |
|---|---|---|---|
| Renal Artery Injection [85] | Rat IRI Model | ➤ Superior long-term engraftment: MSCs persisted in injured kidneys for over 21 days.➤ Reduced off-target localization: Significantly fewer MSCs detected in lungs and spleen compared to IV route.➤ No major procedure-related injuries reported. | ➤ Potent antifibrotic effect: Significantly greater reduction in fibrotic area and α-SMA protein levels vs. IV.➤ Reduced inflammation: Strongest suppression of CD3-positive T-cell infiltration. |
| Intravenous (IV) Injection [85] | Rat IRI Model | ➤ Poor renal retention & lung entrapment: Most cells localized in lungs; few in kidneys, surviving <7 days.➤ Dose-dependent risk: Higher cell doses increase pulmonary embolism risk. | ➤ Modest antifibrotic effect at standard dose.➤ Improved effect with 5x dose, though still inferior to renal artery route. |
| Ultrasound-Guided Renal Subcapsular [86] | Minipig Cisplatin-AKI Model | ➤ Minimally invasive: Avoids vascular complications.➤ High local retention: Creates a localized MSC reservoir.➤ Feasible & safe in a large animal model. | ➤ Promoted functional recovery; multiple transplantations more effective than single.➤ Safe procedure with no major adverse events reported. |
| Supra-Renal Aortic Injection [10] | Human Phase I Trial (Post-Cardiac Surgery) | ➤ Clinically feasible and safe: No adverse events linked to allogeneic MSC administration. | ➤ Reduced post-operative AKI incidence (~20%) compared to historical controls. |
Experimental Protocols for Safety and Efficacy Evaluation
Protocol: Ultrasound-Guided Renal Subcapsular Injection in a Minipig AKI Model
This protocol, adapted from a 2025 study, details a minimally invasive approach designed to maximize local delivery and minimize systemic risks like embolism [86].
Animal Model Preparation:
- Utilize Bama minipigs (15–20 kg).
- Induce AKI via intravenous injection of cisplatin (3.8 mg/kg) combined with a hydration regimen (pre-hydration at 4% body weight, post-hydration at 2% body weight) to ensure animal survival and mimic clinical AKI.
Cell Preparation:
- Use allogeneic MSCs at passages 6-8.
- Prepare cells in saline solution at a concentration of 2 x 10^6 cells/kg for injection.
Ultrasound-Guided Catheterization and Injection:
- Anesthetize the minipig and place it in a prone position.
- Perform routine skin disinfection over the kidney area.
- Using an ultrasound system (e.g., Mindray M9), identify the inferior pole of the kidney and select a puncture site.
- Make a small 3 mm incision at the site with a scalpel.
- Under continuous ultrasound guidance, inject saline through a disposable needle (e.g., 0.7 x 80 mm) to create a space between the renal tissue and the capsule.
- Insert a central venous catheter set (e.g., ARROW ES-04301) through the puncture site into the created subcapsular space.
- Confirm safe catheter placement via ultrasound.
- Slowly inject the MSC suspension (e.g., 5 ml total volume) through the catheter.
- For multiple transplantations, the catheter can be used for repeated injections at 6 hours, 2 days, and 4 days post-AKI induction.
Safety and Efficacy Monitoring:
- Kidney Function: Track serum creatinine (SCr) and blood urea nitrogen (BUN) levels regularly.
- Histological Analysis: At endpoint, perform PAS, Masson, and TUNEL staining on kidney tissues to assess tubular injury, fibrosis, and apoptosis.
- Procedure Safety: Monitor vital signs during and after the procedure. Perform necropsy to check for complications like capsular rupture or hemorrhage.
Protocol: Comparative Analysis of Renal Artery vs. Intravenous Injection
This protocol, based on a 2021 study, allows for a direct comparison of engraftment efficiency and downstream safety and efficacy profiles between two intravascular routes [85].
Animal and Injury Model:
- Use adult rats.
- Induce renal Ischemia-Reperfusion Injury (IRI) by clamping the left renal artery for a defined period (e.g., 45-60 minutes).
Cell Preparation and Labeling:
- Use human bone marrow-derived MSCs (hMSCs).
- Label MSCs with a fluorescent cell tracker (e.g., CM-DiI) for in vivo tracing.
- Resuspend MSCs in PBS at a defined concentration (e.g., 5 x 10^5 cells in 0.2 mL for renal artery/IV; 2.5 x 10^6 cells in 0.3 mL for high-dose IV).
Cell Administration:
- Renal Artery (RA) Group: 60 minutes after reperfusion, cannulate the renal artery and slowly inject the cell suspension (0.2 mL).
- Intravenous (IV) Group: Inject the same cell dose via the inferior vena cava.
- 5x IV Group: Inject a quintuple dose of cells via the inferior vena cava to evaluate dose-dependent effects.
Assessment of Embolism and Engraftment:
- Organ Distribution: At various time points (Days 1, 3, 7, 21), harvest kidneys, lungs, and spleen. Use fluorescence microscopy to quantify CM-DiI-labeled cells and assess pulmonary entrapment.
- Fibrosis and Inflammation: At Day 21 post-IRI, analyze kidney sections with Masson's Trichrome (MT) staining and immunohistochemistry for α-SMA and CD3 to evaluate antifibrotic and anti-inflammatory effects.
Pathway and Workflow Visualization
The following diagram synthesizes the experimental workflow for assessing the safety and efficacy of different MSC delivery routes, as detailed in the protocols above.
The Scientist's Toolkit: Key Research Reagent Solutions
The table below catalogues essential materials and reagents used in the featured protocols for evaluating MSC therapy safety.
Table 2: Essential Research Reagents for MSC Delivery Safety Studies
| Reagent / Material | Function / Application | Example from Literature |
|---|---|---|
| Central Venous Catheter Set | Enables minimally invasive access and injection into the renal subcapsular space in large animals. | ARROW ES-04301 catheter set [86]. |
| Ultrasound Imaging System | Provides real-time guidance for precise needle placement and subcapsular catheterization, minimizing tissue damage. | Mindray M9 system [86]. |
| Fluorescent Cell Tracker (e.g., CM-DiI) | Labels MSCs for in vivo tracking, allowing quantification of cell retention, engraftment duration, and off-target distribution. | Chloromethylbenzamido (CM)-DiI [85]. |
| Cisplatin | A chemotherapeutic agent used to induce a stable and reproducible nephrotoxic AKI model in rodents and large animals. | Cisplatin injection (e.g., Nuoxin) [86]. |
| Antibodies for IHC | Used to quantify key safety and efficacy endpoints, including fibrosis (α-SMA), inflammation (CD3), and tubular injury (KIM-1). | Antibodies against α-SMA, CD3, KIM-1 [86] [85]. |
| Automatic Biochemical Analyzer | Standardized measurement of renal function biomarkers (Serum Creatinine, Blood Urea Nitrogen) to assess functional recovery. | Roche cobas 8000 system [86]. |
The evaluation of acute kidney injury (AKI) has evolved beyond traditional functional markers like serum creatinine and urine output, which primarily indicate reduced glomerular filtration but offer limited insight into the timing, site, or mechanism of tubular injury [87] [88]. The discovery and validation of damage-specific biomarkers, including Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Kidney Injury Molecule-1 (KIM-1), enable earlier detection and more precise stratification of AKI [88]. In research focused on renal cortex injection of mesenchymal stem cells (MSCs) for paracrine therapy, these biomarkers provide critical tools for quantifying the efficacy and timing of therapeutic interventions. They allow researchers to monitor the specific molecular responses to MSC-secreted factors, which promote repair through anti-apoptotic, anti-inflammatory, and antioxidant pathways [11] [3]. By correlating dynamic changes in biomarker levels with functional and histological outcomes, scientists can better delineate the therapeutic mechanisms of MSCs and optimize treatment protocols.
AKI Biomarker Profiles: Characteristics and Interpretive Frameworks
Classification and Physiological Roles of Key AKI Biomarkers
AKI biomarkers can be categorized based on their origin and physiological function, providing a framework for interpreting their diagnostic and prognostic significance [88].
- Functional Biomarkers: These reflect the glomerular filtration rate (GFR).
- Serum Creatinine (sCr): A gold standard functional marker, but levels can be influenced by non-renal factors like muscle mass, fluid balance, and certain drugs, leading to delayed AKI diagnosis [88].
- Cystatin C: A functional marker less dependent on muscle mass than sCr, offering a potentially more reliable GFR estimate [88].
- Damage/Stress Biomarkers: These indicate direct injury to renal tubular cells.
- NGAL (Neutrophil Gelatinase-Associated Lipocalin): Upregulated in distal tubular epithelial cells in response to injury. It appears rapidly in urine and plasma following various AKI insults and is useful for early diagnosis [87] [88].
- KIM-1 (Kidney Injury Molecule-1): A transmembrane protein highly upregulated in proximal tubular cells following injury. It plays a role in phagocytic clearance of damaged cells and is a specific marker of proximal tubular damage [87] [88].
- TIMP-2 & IGFBP7 (Tissue Inhibitor of Metalloproteinases-2 & Insulin-like Growth Factor-Binding Protein 7): These markers are involved in G1 cell cycle arrest, a mechanism enacted by cells under stress. Their product, [TIMP-2]•[IGFBP7], is a sensitive indicator of tubular stress and predicts the development of moderate to severe AKI [88].
- L-FABP (Liver-type Fatty Acid-Binding Protein): Expressed in proximal tubular cells, it is released into urine during oxidative stress [87].
- IL-18 (Interleukin-18): A pro-inflammatory cytokine cleaved by caspase-1 and released from tubular epithelial cells, serving as a marker of intra-renal inflammation [87] [88].
Quantitative Biomarker Profiles in AKI Diagnosis and Prognosis
The following table summarizes key characteristics and reported performance metrics for prominent AKI biomarkers, providing a reference for experimental planning and data interpretation.
Table 1: Key Biomarkers for Acute Kidney Injury Assessment
| Biomarker | Full Name | Primary Source | Biological Role | Clinical Utility | Representative Performance (AUC) & Context |
|---|---|---|---|---|---|
| NGAL | Neutrophil Gelatinase-Associated Lipocalin | Distal Tubular Cells, Neutrophils | Binds bacterial siderophores; inflammatory response | Early AKI diagnosis, prognosis | AUC 0.75-0.80 for early AKI prediction in critical illness [88] |
| KIM-1 | Kidney Injury Molecule-1 | Proximal Tubular Cells | Phagocytosis of apoptotic cells; specific tubular damage | Specific marker for proximal tubular injury | Higher levels correlate with severity and adverse outcomes [87] |
| [TIMP-2]•[IGFBP7] | Tissue Inhibitor of Metalloproteinases-2 • Insulin-like Growth Factor-Binding Protein 7 | Tubular Cells (constitutive) | G1 cell cycle arrest in response to cellular stress | Risk stratification for severe AKI | AUC 0.80-0.85 for predicting moderate-severe AKI within 12-24 hours [88] |
| L-FABP | Liver-type Fatty Acid-Binding Protein | Proximal Tubular Cells | Binds fatty acids; response to oxidative stress | Marker of oxidative stress in proximal tubules | Elevated in AKI; levels correlate with oxidative damage [87] |
| IL-18 | Interleukin-18 | Proximal Tubular Cells, Macrophages | Pro-inflammatory cytokine | Marker of intra-renal inflammation | Predictive of AKI in mixed clinical settings [87] |
| CCL14 | C-C Motif Chemokine Ligand 14 | Monocytes, Macrophages | Leukocyte recruitment and activation | Prognosis; identifies persistent AKI | High levels associated with increased risk of dialysis or death [88] |
An Integrated Framework for Biomarker Interpretation
A consensus approach recommended by the Acute Dialysis Quality Initiative (ADQI) involves simultaneously assessing functional (e.g., sCr) and damage (e.g., NGAL, KIM-1) biomarkers. This creates a two-axis framework that classifies patients into one of four quadrants, enabling a more nuanced understanding of their AKI status [87]:
- No AKI: No change in functional or damage markers.
- Subclinical AKI (Kidney Damage): Elevated damage markers without a rise in sCr. This state carries an increased risk of subsequent adverse events [87] [88].
- Functional AKI: A rise in sCr without elevated damage markers, potentially representing hemodynamic alterations without significant tubular injury (e.g., in prerenal azotemia) [88].
- Established AKI (Damage + Dysfunction): Elevations in both functional and damage markers, confirming significant structural injury with functional impairment.
This framework is particularly valuable in MSC therapy research, as it can help distinguish between a mere functional effect and a genuine mitigation of tubular damage by the treatment.
Application Notes: Correlating Biomarker Dynamics with MSC Paracrine Therapy
Biomarkers as Measures of MSC Efficacy
In models of AKI, the therapeutic effect of MSCs is largely mediated by their paracrine secretion of bioactive molecules, which exert anti-apoptotic, antioxidant, and immunomodulatory effects [11] [3]. Tracking specific damage biomarkers provides a quantitative method to validate these mechanisms.
- Anti-apoptotic Effects: MSC administration has been shown to significantly reduce expression of pro-apoptotic markers like Bax and cleaved caspase-3, while increasing the anti-apoptotic protein Bcl-2. This reduction in tubular cell death is directly correlated with falling levels of KIM-1 and NGAL, indicating a decrease in ongoing tubular injury [3].
- Antioxidant Effects: MSCs can reduce oxidative stress, as measured by a decrease in urinary 8-hydroxy-2'-deoxyguanosine (8-OHdG) and an upregulation of endogenous antioxidant enzymes like glutathione peroxidase (GPx) and catalase. This reduction in oxidative stress parallels the decline in damage biomarkers [3].
- Immunomodulation: MSCs and their extracellular vesicles (MSC-EVs) can polarize renal macrophages from a pro-inflammatory (M1) to a reparative (M2) phenotype. This modulation of the inflammatory microenvironment contributes to renal repair, a process that can be indirectly monitored by changes in inflammatory biomarkers like IL-18 [52].
Experimental Protocol: Validating MSC Therapy in Rodent AKI Model
The following protocol details a standard methodology for inducing gentamicin (GM)-induced AKI in rats and evaluating the therapeutic effect of MSC administration through functional and damage biomarkers.
Objective: To evaluate the efficacy of tonsil-derived mesenchymal stem cells (T-MSCs) in ameliorating gentamicin-induced AKI in a rodent model by assessing renal function, tubular damage, apoptosis, and oxidative stress.
Materials:
- Animals: Male Sprague-Dawley rats (200-250 g)
- AKI Inducing Agent: Gentamicin (GM)
- Therapeutic Cells: Tonsil-derived MSCs (T-MSCs) [3]
- Key Reagents:
- PKH26 fluorescent dye for cell tracking
- Assay kits for BUN and creatinine
- Antibodies for Bax, Bcl-2, cleaved caspase-3, KIM-1, NGAL, GPx, catalase
- TUNEL assay kit for apoptosis detection
- ELISA kit for urinary 8-OHdG
Procedure:
- AKI Induction and Treatment Groups:
- Randomly assign rats to four groups (n=5/group): Control (vehicle), T-MSC (cells only), GM (injury only), GM + T-MSC (therapy) [3].
- Induce AKI via daily intraperitoneal injections of GM (70 mg/kg) for 10 days [3].
- On day 11, administer a single intravenous injection of T-MSCs (1 × 10⁷ cells) via the tail vein to the GM+T-MSC group. The GM group receives an injection of vehicle/PBS [3].
- Sample Collection:
- On day 16, sacrifice animals and collect blood, urine, and kidney tissue.
- Process blood for serum and preserve kidney tissue for histology (paraffin embedding) and protein analysis (snap-freezing) [3].
- Renal Function and Damage Assessment:
- Functional Biomarkers: Quantify serum Blood Urea Nitrogen (BUN) and creatinine using commercial assay kits [3].
- Histological Damage: Perform Periodic Acid-Schiff (PAS) staining on kidney sections. Score tubular injury (0-4) based on the percentage of damaged tubules exhibiting necrosis, cast formation, and dilation [3].
- Tubular Damage Biomarkers: Analyze tissue expression of KIM-1 and NGAL via immunohistochemistry or Western blot [3].
- Mechanistic Biomarker Analysis:
- Apoptosis: Perform TUNEL staining on kidney sections and calculate the apoptotic ratio. Corroborate with Western blot analysis of Bax, Bcl-2, and cleaved caspase-3 [3].
- Oxidative Stress: Measure urinary 8-OHdG by ELISA. Assess renal expression of antioxidant enzymes GPx and catalase by Western blot [3].
- Cell Tracking:
- Prior to injection, label T-MSCs with PKH26 fluorescent dye. After sacrifice, visualize labeled cells in kidney cortex sections using fluorescence microscopy to confirm engraftment [3].
Table 2: Experimental Parameters for Rodent AKI Model
| Parameter | Method of Assessment | Group Comparison (GM vs. GM+T-MSC) | Interpretation |
|---|---|---|---|
| Renal Function | BUN & Creatinine Assays | Decrease in GM+T-MSC | Improved glomerular filtration |
| Structural Damage | PAS Staining & Scoring | Lower tubular injury score in GM+T-MSC | Reduced histological damage |
| Tubular Injury | KIM-1/NGAL IHC/Western Blot | Reduced expression in GM+T-MSC | Attenuation of specific tubular damage |
| Apoptosis | TUNEL Staining; Apoptotic Protein WB | Fewer TUNEL+ cells; ↓Bax/cleaved caspase-3, ↑Bcl-2 in GM+T-MSC | Anti-apoptotic effect of therapy |
| Oxidative Stress | Urinary 8-OHdG ELISA; GPx/Catalase WB | ↓8-OHdG; ↑GPx/Catalase in GM+T-MSC | Reduction in oxidative damage |
Signaling Pathways in MSC-Mediated Renoprotection
The therapeutic benefits of MSCs in AKI are mediated through complex signaling pathways that modulate inflammation, apoptosis, and oxidative stress. The following diagram illustrates a key pathway involving MSC-EVs and macrophage polarization.
Diagram 1: MSC-EVs promote macrophage polarization and renal repair via TXNIP-NFκB signaling. Extracellular vesicles from adipose-derived MSCs (AMSC-EVs) inhibit TXNIP expression, which downregulates the pro-inflammatory IKKα/NF-κB pathway. This suppression promotes the polarization of renal CX3CR1+ macrophages towards a reparative M2 phenotype, facilitating tissue repair [52].
The Scientist's Toolkit: Essential Reagents for AKI Biomarker and MSC Research
Table 3: Essential Research Reagents for AKI Biomarker and MSC Therapy Studies
| Category | Item | Function & Application | Example Context |
|---|---|---|---|
| AKI Modeling | Gentamicin | Aminoglycoside antibiotic; induces predictable proximal tubular injury in rodents. | In vivo model of nephrotoxic AKI [3]. |
| Cisplatin | Chemotherapeutic agent; induces oxidative stress and apoptosis in tubular cells. | In vivo and in vitro models of AKI [52]. | |
| Cell Therapy | Mesenchymal Stem Cells (MSCs) | Primary therapeutic agent; sourced from bone marrow, adipose tissue, or tonsils. | Paracrine-mediated renoprotection [11] [3]. |
| MSC-EVs (Extracellular Vesicles) | Cell-free therapeutic alternative; carries bioactive molecules from MSCs. | Attenuates AKI via macrophage polarization [52]. | |
| Biomarker Assays | ELISA Kits | Quantifies protein levels of biomarkers (NGAL, KIM-1, IL-18, 8-OHdG) in urine/serum/tissue. | Objective measurement of tubular injury and stress [3] [88]. |
| Antibodies (IHC/Western) | Detects and visualizes biomarker distribution and expression (KIM-1, NGAL, Bax, Bcl-2). | Histological and protein-level analysis of injury mechanisms [3]. | |
| Cell Tracking | PKH26 / DiI Lipophilic Dyes | Fluorescently labels cell membranes for in vivo tracking of administered MSCs. | Visualizing MSC localization and retention in kidney tissue [3]. |
| Pathway Analysis | Antibodies (Phospho-specific) | Targets specific phosphorylated proteins to assess activity of signaling pathways (e.g., NF-κB). | Mechanistic studies on TXNIP-IKKα/NF-κB pathway [52]. |
Acute Kidney Injury (AKI) represents a significant global health burden, affecting approximately 13.3 million people annually worldwide, with mortality rates reaching 20-50% in severe cases [11] [89]. The therapeutic potential of Mesenchymal Stem Cells (MSCs) for AKI has garnered substantial scientific interest due to their regenerative, immunomodulatory, and anti-inflammatory capabilities [11] [2]. However, the clinical translation of MSC-based therapies faces considerable challenges, primarily due to low cell retention and poor survival rates when administered via conventional systemic routes [11] [2].
The limitations of systemic delivery have prompted investigation into local administration strategies. Intravenous (IV) administration, while minimally invasive, results in significant entrapment of MSCs in pulmonary capillary beds—a phenomenon known as the "pulmonary first-pass effect"—with the liver serving as another major site of sequestration [2]. This widespread distribution leads to subtherapeutic MSC concentrations at the actual site of renal injury. Intra-arterial (IA) delivery can bypass pulmonary filtration but carries risks of cell embolism and increased procedural trauma [2]. In contrast, local delivery methods, particularly renal cortex injection, offer a promising alternative by depositing MSCs directly into the target tissue, thereby maximizing engraftment while minimizing systemic exposure and potential complications [2].
Current Clinical Trial Landscape for MSC Therapy in AKI
The clinical investigation of MSC therapy for AKI is ongoing, with several trials exploring various delivery routes and patient populations. The table below summarizes key registered clinical trials based on available data:
Table 1: Clinical Trials of MSC Therapy for Acute Kidney Injury
| Registration Number | Status | Study Title | Interventions | Country |
|---|---|---|---|---|
| NCT00733876 | Completed | Allogeneic Multipotent Stromal Cell Treatment for Acute Kidney Injury Following Cardiac Surgery [90] | Multipotent stromal cells | USA |
| NCT01602328 | Terminated | A Study to Evaluate the Safety and Efficacy of AC607 for the Treatment of Kidney Injury in Cardiac Surgery Subjects [90] | AC607 (allogeneic MSCs) | USA |
| NCT03015623 | Active, not recruiting | A Study of Cell Therapy for Subjects With Acute Kidney Injury Who Are Receiving Continuous Renal Replacement Therapy [90] | SBI-101 (extracorporeal stromal cell therapeutic) | USA |
| NCT04194671 | Not yet recruiting | Clinical Trial of Mesenchymal Stem Cells in the Treatment of Severe Acute Kidney Injury [90] | Mesenchymal stem cells | China |
| NCT04445220 | Recruiting | A Study of Cell Therapy in COVID-19 Subjects With Acute Kidney Injury Who Are Receiving Renal Replacement Therapy [90] | SBI-101 | USA |
Most current clinical trials favor systemic intravenous infusion. However, a recent patient-blinded, randomized, placebo-controlled trial in China (NCT04194671) is investigating two IV infusions of umbilical cord-derived MSCs on days 0 and 7 for severe AKI patients [90]. This design highlights the exploration of repeated dosing to potentially overcome the limitations of single systemic administrations. Notably, the field has yet to register a clinical trial specifically investigating local renal delivery for AKI, indicating a significant opportunity for clinical development in this area.
Preclinical Evidence Supporting Local Renal Delivery
Preclinical studies in animal models provide compelling evidence for the superior efficacy of local MSC delivery compared to systemic routes. These studies demonstrate not only improved functional and structural recovery but also elucidate the underlying mechanisms of action.
Table 2: Preclinical Studies of Local MSC Delivery in AKI Models
| Delivery Method | Animal Model | MSC Source | Key Findings | Reference |
|---|---|---|---|---|
| Local Renal Injection | Murine UUO (CKD) model | Human Adipose-Derived (Pr-MSCs) | Reduced collagen deposition and increased expression of the anti-inflammatory cytokine IL-10; Systemic administration showed no significant effect. [37] | [37] |
| Local Renal Injection | Rat IRI model | Adipose-Derived (in Hydrogel) | Significantly improved renal function and ameliorated tubular injury; Hydrogel enhanced cell retention and viability. [2] | [2] |
| Intravenous (Comparison) | Rat Gentamicin-induced AKI | Tonsil-Derived (T-MSCs) | T-MSCs localized in tubules, exerting anti-apoptotic & anti-oxidative effects; demonstrates homing potential even with IV delivery. [3] | [3] |
A comparative study in a murine unilateral ureteral obstruction (UUO) model directly assessed delivery routes, finding that local deliveries of preconditioned MSCs significantly reduced collagen deposition and increased expression of the anti-inflammatory cytokine IL-10, whereas systemic administration of the same cells showed no significant effect on UUO-induced injury [37]. This stark contrast underscores the therapeutic advantage of local application.
The mechanism of benefit extends beyond direct physical incorporation. Research on tonsil-derived MSCs (T-MSCs) delivered intravenously in gentamicin-induced AKI demonstrated that MSCs can localize within damaged renal tubules and exert potent anti-apoptotic and anti-oxidative effects, reducing markers like Bax and cleaved caspase while enhancing expression of glutathione peroxidase and catalase [3]. This suggests that once the challenge of initial delivery and retention is overcome via local injection, MSCs can powerfully modulate the local tissue microenvironment to promote repair.
Optimizing MSC Therapeutic Potential: Preconditioning and 3D Culture
To enhance MSC efficacy upon delivery, various optimization strategies have been developed, focusing on boosting cell potency and resilience.
Preconditioning Strategies
Preconditioning exposes MSCs to sublethal stress or bioactive factors prior to administration, priming them for the harsh microenvironment of injured tissue.
Table 3: Preconditioning Strategies to Enhance MSC Efficacy
| Preconditioning Method | Specific Treatment | Proposed Mechanism of Action | Effect in AKI Models | Reference |
|---|---|---|---|---|
| Hypoxic Preconditioning | 1-5% O₂ or CoCl₂ | Enhances secretion of pro-angiogenic (VEGF, HGF) and homing (CXCR4) factors; improves survival & migration. [11] | Improved renal function, reduced apoptosis, enhanced angiogenesis in IRI models. [11] | [11] |
| Cytokine Preconditioning | TNF-α & IFN-γ | Promotes secretion of immunomodulatory factors (PGE2, IDO), enhancing anti-inflammatory and anti-fibrotic capacity. [37] | Preconditioned MSCs (Pr-MSCs) showed superior anti-fibrotic effects in a UUO model. [37] | [37] |
| Drug Preconditioning | Chlorzoxazone (CZ) | Induces an anti-inflammatory phenotype in MSCs by promoting FoxO3 phosphorylation, boosting immunosuppressive function. [11] | Attenuated renal inflammation and glomerular damage in antibody-induced AKI. [11] | [11] |
Three-Dimensional (3D) Culture Systems
Transitioning from traditional 2D monolayer culture to 3D systems (e.g., spheroids, hydrogels) better mimics the native cellular microenvironment, preventing senescence and preserving stem cell functionality [2]. Hydrogels, in particular, serve a dual purpose as 3D culture platforms and delivery vehicles.
Hydrogel-Focused Strategies:
- Natural Polymer Hydrogels (e.g., Alginate, Chitosan, Hyaluronic Acid): Offer high biocompatibility and biodegradability. Alginate reinforced with hyaluronic acid shows promise for AKI intervention, though pure alginate has limitations in cell adhesion [2].
- Synthetic Polymer Hydrogels (e.g., PEG, PLA): Provide superior tunability of mechanical properties and degradation rates but may require modification with bioactive motifs (e.g., RGD peptides) to enhance cell interaction [2].
The following diagram illustrates the logical workflow for optimizing and delivering MSCs for AKI therapy, incorporating preconditioning, 3D culture, and the final local application.
Diagram: Optimization and Local Delivery Workflow for MSCs in AKI. This workflow outlines the key steps from cell isolation to the final therapeutic outcome, highlighting critical optimization nodes.
Detailed Experimental Protocol: Renal Cortex Injection of MSCs in Preclinical Models
This protocol provides a detailed methodology for the local renal delivery of MSCs in a rodent model of AKI, incorporating best practices from the literature.
Materials and Reagents
Table 4: Research Reagent Solutions for MSC Renal Injection
| Item | Function/Description | Example/Specification |
|---|---|---|
| MSCs | Therapeutic agent. | Human Umbilical Cord MSCs (uc-MSCs), passages 3-8 [90]. Characterized by CD73+, CD90+, CD105+, CD34-, CD45- [89]. |
| Hydrogel | 3D delivery vehicle to enhance cell retention and survival. | Natural polymer (e.g., Alginate-Hyaluronic Acid composite) or synthetic (e.g., RGD-modified PEG) [2]. |
| Preconditioning Media | Enhances MSC therapeutic potency prior to injection. | Advanced MEM serum-free media supplemented with cytokines (e.g., 10 ng/mL TNF-α & IFN-γ) [37]. |
| Anesthetics | Surgical anesthesia and peri-operative analgesia. | Ketamine/Xylazine or Isoflurane. Approved by Institutional Animal Care and Use Committee (IACUC). |
| Antibiotics | Post-operative prophylaxis against infection. | e.g., Enrofloxacin. |
| Surgical Instruments | For performing the surgical procedure. | Fine scissors, forceps, retractors, hemostats, and a 30-gauge insulin syringe for injection. |
| Analgesics | Post-operative pain management. | e.g., Buprenorphine. |
Step-by-Step Procedure
MSC Preparation and Preconditioning:
- Culture MSCs in standard flasks until 70-90% confluency.
- For preconditioning, culture MSCs for 24 hours in serum-free media supplemented with preconditioning agents (e.g., 10 ng/mL TNF-α and IFN-γ) [37].
- On the day of surgery, harvest MSCs using 0.25% trypsin, wash, and resuspend in PBS or mix with a hydrogel vehicle (e.g., 1x10^7 cells/mL in sterile, cold alginate solution) [3] [2]. Keep the cell suspension on ice until implantation.
Animal Preparation and Surgical Exposure:
- Anesthetize the rodent (e.g., Sprague-Dawley rat) using an approved anesthetic regimen.
- Secure the animal in a lateral decubitus position. Shave and aseptically prepare the surgical area (dorsolateral flank).
- Make a ~1.5 cm oblique incision in the skin and muscle layer along the costovertebral angle.
- Gently expose the kidney using moistened sterile cotton swabs. Place a sterile gauze pad around the surgical field.
Renal Cortex Injection:
- Load the cell suspension (e.g., 1x10^6 to 2x10^6 cells in 50-100 µL total volume) into a 1 mL insulin syringe with a 30-gauge needle [2].
- Gently stabilize the kidney. Insert the needle bevel-up at a shallow angle (~10-15 degrees) into the renal cortex.
- Slowly depress the plunger to inject the solution. A superficial, blanching bleb indicates a successful intraparenchymal injection.
- Hold the needle in place for 10-15 seconds post-injection to prevent reflux.
- Withdraw the needle carefully and apply gentle pressure with a sterile cotton tip if minor bleeding occurs.
Closure and Post-operative Care:
- Return the kidney to its anatomical position.
- Suture the muscle layer with absorbable suture material and close the skin with wound clips or non-absorbable sutures.
- Administer analgesics (e.g., Buprenorphine) and antibiotics (e.g., Enrofloxacin) subcutaneously according to institutional protocols.
- Monitor animals until fully recovered from anesthesia and daily thereafter for signs of distress or infection.
Key Technical Considerations
- Injection Volume and Site: Limit injection volume to 50-100 µL per kidney to avoid tissue damage and significant reflux. Target the subcapsular cortex, which is rich in tubular structures [2].
- Cell Viability: Maintain high cell viability (>95%) throughout the harvesting and injection process. Using a hydrogel vehicle can significantly improve post-transplantation survival [2].
- Control Groups: Essential control groups include: 1) Sham-operated (surgery, no AKI, no cells), 2) Vehicle control (AKI + delivery vehicle only), and 3) Systemically treated group (AKI + IV MSCs) for direct comparison [37].
Regulatory and Trial Design Considerations
Designing a first-in-human clinical trial for local renal delivery of MSCs requires careful planning and early regulatory engagement.
Engaging with Regulatory Bodies: The FDA's Complex Innovative Trial Design (CID) Paired Meeting Program provides a platform for sponsors to discuss novel clinical trial designs, including those for innovative delivery methods. This program is suitable for proposals beyond first-in-human studies, where there is sufficient clinical information to inform the novel design [91].
Key Design Elements for a CID Proposal: A successful proposal should include a strong rationale for local delivery, a detailed study schema, defined endpoints (e.g., change in creatinine over 28 days [90]), a rigorous statistical analysis plan, and comprehensive simulations demonstrating the trial's operating characteristics (type I error, power) [91].
Safety Monitoring: A local delivery trial must include a dedicated Data and Safety Monitoring Board (DSMB) and detailed stopping rules. Potential risks specific to renal cortex injection (e.g., bleeding, perforation, hydronephrosis) require close monitoring.
Conclusion
Renal cortex injection represents a significant methodological advancement for MSC-based AKI therapy by directly addressing the critical limitations of systemic administration. This targeted approach enhances MSC retention and survival at the injury site, thereby amplifying their paracrine therapeutic effects. When combined with optimization strategies such as preconditioning, 3D culture, and genetic modification, localized delivery maximizes the potential of MSCs to modulate immune responses, reduce oxidative stress, and promote tubular regeneration. While preclinical evidence is compelling, successful clinical translation will require standardized protocols, refined delivery techniques, and well-designed clinical trials that specifically evaluate the safety and efficacy of renal cortex injection. Future research should focus on developing minimally invasive delivery systems, identifying optimal MSC sources, and establishing patient selection criteria to fully realize the potential of this promising therapeutic strategy for AKI.
References
- 1. Cell therapy in kidney diseases: advancing treatments for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11669058/]
- 2. Optimization strategies of mesenchymal stem cell-based ... [https://stemcellres.biomedcentral.com/articles/10.1186/s13287-023-03351-2]
- 3. Tonsil-derived mesenchymal stem cells protect the kidney ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12611628/]
- 4. Molecular Mechanisms of Mesenchymal Stem Cell-Based ... [https://www.mdpi.com/1422-0067/22/21/11406]
- 5. Incidence and Risk Factors for Progression of Acute Kidney ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12410291/]
- 6. Chronic kidney disease and the global public health agenda [https://www.nature.com/articles/s41581-024-00820-6]
- 7. Immunomodulatory effects of mesenchymal stem cell therapy ... [https://bmcnephrol.biomedcentral.com/articles/10.1186/s12882-025-04029-y]
- 8. Potential Therapeutic Effect and Mechanisms of ... [https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2022.824752/full]
- 9. Mesenchymal stem cells in acute kidney injury - PubMed - NIH [https://pubmed.ncbi.nlm.nih.gov/17914926/]
- 10. Mesenchymal Stem Cell Treatment for Ischemic Kidney ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3795813/]
- 11. International Journal of Molecular Medicine [https://www.spandidos-publications.com/10.3892/ijmm.2025.5620?text=fulltext]
- 12. AMSC-EVs attenuate acute kidney injury through TXNIP-IKKα ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12619428/]
- 13. Worldwide hotspots and trends in stem cell therapy for kidney ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12318756/]
- 14. Molecular Mechanisms of Mesenchymal Stem Cell-Based ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8583897/]
- 15. Secretomes from Mesenchymal Stem Cells against Acute ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6311717/]
- 16. Mesenchymal stem cell-derived extracellular vesicles for renal ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5628022/]
- 17. Mesenchymal stem cell-derived extracellular vesicles for ... [https://www.nature.com/articles/s41419-022-05034-x]
- 18. Mesenchymal Stromal Cell Uses for Acute Kidney Injury— ... [https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2020.01369/full]
- 19. Mesenchymal stromal cells secretome restores bioenergetic ... [https://stemcellres.biomedcentral.com/articles/10.1186/s13287-023-03563-6]
- 20. Mechanism of mesenchymal stem cells in treating diabetic ... [https://stemcellres.biomedcentral.com/articles/10.1186/s13287-025-04750-3]
- 21. Inflammatory priming of mesenchymal stromal cells ... [https://www.sciencedirect.com/science/article/pii/S0006291X25011064]
- 22. Mesenchymal stem cells in treating human diseases [https://www.nature.com/articles/s41392-025-02313-9]
- 23. Mesenchymal stem cell therapy targeting mitochondrial dysfunction in acute kidney injury | Journal of Translational Medicine | Full Text [https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-019-1893-4]
- 24. Mitochondrial metabolism and targeted treatment ... [https://www.nature.com/articles/s41420-024-01843-5]
- 25. Mesenchymal stromal cell-mediated mitochondrial transfer ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12505854/]
- 26. Mitochondrial Transfer as a Novel Therapeutic Approach in Disease Diagnosis and Treatment - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10218908/]
- 27. Cisplatin-induced acute kidney injury is alleviated by ... [https://www.sciencedirect.com/science/article/pii/S2352320425001841]
- 28. Targeting mitochondrial transfer as a promising therapeutic strategy - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S1471491425000899]
- 29. [Growth factors and renal regeneration] [https://pubmed.ncbi.nlm.nih.gov/20651879/]
- 30. Growth factors and renal regeneration - Nefrología [https://revistanefrologia.com/en-growth-factors-renal-regeneration-articulo-X2013251410050516]
- 31. Trophic Factors from Tissue Stem Cells for Renal Regeneration [https://pmc.ncbi.nlm.nih.gov/articles/PMC4452108/]
- 32. The Growth Factors: Potential Biomarkers and Therapeutic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9710479/]
- 33. Potential targeted therapy and diagnosis based on novel ... [https://www.nature.com/articles/s41392-020-0106-1]
- 34. Genomic meta-analysis of growth factor and integrin pathways ... [https://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-14-275]
- 35. Optimization strategies of mesenchymal stem cell-based ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10150535/]
- 36. Pharmacokinetic characteristics of mesenchymal stem cells ... [https://www.nature.com/articles/s41392-024-01936-8]
- 37. Comparative study of systemic and local delivery ... [https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2024.1456416/full]
- 38. Stem cell delivery to kidney via minimally invasive ... [https://www.nature.com/articles/s41598-020-64417-2]
- 39. Mesenchymal stem cells therapy for acute kidney injury [https://pmc.ncbi.nlm.nih.gov/articles/PMC10133560/]
- 40. Mesenchymal stromal cell–based therapies for acute ... [https://www.sciencedirect.com/science/article/pii/S0085253820300934]
- 41. Mesenchymal Stem Cell-Based Therapy for Kidney Disease [https://pmc.ncbi.nlm.nih.gov/articles/PMC5046016/]
- 42. Immunomodulatory effects of mesenchymal stem cell ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11874639/]
- 43. The Beneficial Effects of Mesenchymal Stem Cells in Acute ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10680085/]
- 44. Mesenchymal Stem Cells as Anti-Inflammatory Agents in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12428256/]
- 45. Ex vivo live cell tracking in kidney organoids using light sheet ... [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0199918]
- 46. Dyna-vivo-seq unveils cellular RNA dynamics during acute ... [https://www.nature.com/articles/s41467-024-54202-4]
- 47. Novel in vivo techniques to visualize kidney anatomy and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4490063/]
- 48. Renal Artery Administration with Optimal Numbers [https://pmc.ncbi.nlm.nih.gov/articles/PMC3956922/]
- 49. Cisplatin-induced acute kidney injury is alleviated by ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12424231/]
- 50. Tonsil-derived mesenchymal stem cells protect ... [https://www.krcp-ksn.org/m/journal/view.php?number=6487]
- 51. Biological Membrane-Packed Mesenchymal Stem Cells ... [https://www.nature.com/articles/srep41136]
- 52. AMSC-EVs attenuate acute kidney injury through TXNIP-IKKα ... [https://stemcellres.biomedcentral.com/articles/10.1186/s13287-025-04754-z]
- 53. Fiducial Marker Placement [https://www.radiologyinfo.org/en/info/fiducial-marker]
- 54. All-trans retinoic acid pretreatment of mesenchymal stem cells ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11129434/]
- 55. Mesenchymal stem cells suppress kidney injury molecule ... [https://www.archivesofmedicalscience.com/Mesenchymal-Stem-Cells-Suppress-Kidney-Injury-molecule-1-Associated-with-Inhibition,190868,0,2.html]
- 56. Bone marrow mesenchymal stem cells-derived exosomes ... [https://www.nature.com/articles/s41598-025-25204-z]
- 57. Novel preconditioning strategies for enhancing the migratory ability of mesenchymal stem cells in acute kidney injury - PubMed [https://pubmed.ncbi.nlm.nih.gov/30139368/]
- 58. Preconditioning strategies for improving the survival rate and paracrine ability of mesenchymal stem cells in acute kidney injury - PubMed [https://pubmed.ncbi.nlm.nih.gov/30484934/]
- 59. Hypoxic Preconditioning with Cobalt of Bone Marrow Mesenchymal Stem Cells Improves Cell Migration and Enhances Therapy for Treatment of Ischemic Acute Kidney Injury | PLOS One [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0062703]
- 60. Hypoxic mesenchymal stem cells ameliorate acute kidney ischemia-reperfusion injury via enhancing renal tubular autophagy | Stem Cell Research & Therapy | Full Text [https://stemcellres.biomedcentral.com/articles/10.1186/s13287-021-02374-x]
- 61. Comparative Analysis of 3D Culture Methodologies in ... - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC11952452/]
- 62. Preventing MSC aging and enhancing immunomodulation [https://www.sciencedirect.com/science/article/pii/S2352320425000902]
- 63. The secretion profile of mesenchymal stem cells and ... [https://www.nature.com/articles/s41392-022-00932-0]
- 64. Paracrine effects of mesenchymal stem cells in cisplatin ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3023217/]
- 65. A brief review of biomaterials used in drug delivery, vaccine ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8958282/]
- 66. Stem cells: a potential treatment option for kidney diseases [https://stemcellres.biomedcentral.com/articles/10.1186/s13287-020-01751-2]
- 67. Creation and implantation of acellular rat renal ECM-based ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4594518/]
- 68. Platelet-rich plasma promotes MSCs exosomes paracrine to ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8014389/]
- 69. Current Perspectives on Role of MSC in Renal Pathophysiology [https://pmc.ncbi.nlm.nih.gov/articles/PMC6158317/]
- 70. Enhancing the Therapeutic Potential of Mesenchymal ... [https://www.mdpi.com/1422-0067/23/11/6035]
- 71. Iron-Quercetin complex enhances mesenchymal stem cell ... [https://www.sciencedirect.com/science/article/pii/S2352320424002232]
- 72. Ultrasound-guided renal subcapsular transplantation of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11871648/]
- 73. Effect of injecting adipose stem cells combined with platelet ... [https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2025.1409056/full]
- 74. Therapeutic effect of umbilical cord mesenchymal stem ... [https://pubmed.ncbi.nlm.nih.gov/40208785/]
- 75. Tonsil-derived mesenchymal stem cells protect the kidney ... [https://pubmed.ncbi.nlm.nih.gov/40905041/]
- 76. Beyond preclinical promise: can mesenchymal stromal cell ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12540260/]
- 77. Cell therapy in kidney diseases: advancing treatments for ... [https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2024.1505601/full]
- 78. Histopathological and Immunohistochemical Study of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11326785/]
- 79. A Systematic Review of Clinical Characteristics and Histologic ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7609907/]
- 80. Histology, Staining - StatPearls - NCBI Bookshelf - NIH [https://www.ncbi.nlm.nih.gov/books/NBK557663/]
- 81. H&E Staining Overview: A Guide to Best Practices [https://www.leicabiosystems.com/us/knowledge-pathway/he-staining-overview-a-guide-to-best-practices/]
- 82. Quantitative analysis of histopathological findings using ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5660959/]
- 83. Mesenchymal Stem Cells Delivered Locally to Ischemia ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10340256/]
- 84. Comparison of the treatment efficacy of umbilical ... [https://stemcellres.biomedcentral.com/articles/10.1186/s13287-022-02805-3]
- 85. Localization and Maintenance of Engrafted Mesenchymal ... [https://www.mdpi.com/1422-0067/22/8/4178]
- 86. Ultrasound-guided renal subcapsular transplantation of ... [https://stemcellres.biomedcentral.com/articles/10.1186/s13287-025-04137-4]
- 87. Current Use of Biomarkers in Acute Kidney Injury - PMC - NIH [https://pmc.ncbi.nlm.nih.gov/articles/PMC4198530/]
- 88. Biomarkers in acute kidney injury - Annals of Intensive Care [https://annalsofintensivecare.springeropen.com/articles/10.1186/s13613-024-01360-9]
- 89. Narrative Review of Mesenchymal Stem Cell Therapy in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11655143/]
- 90. Efficacy of umbilical cord mesenchymal stem cell transfusion ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8862499/]
- 91. Complex Innovative Trial Design Meeting Program [https://www.fda.gov/drugs/development-resources/complex-innovative-trial-design-meeting-program]