Research Articles
Designing Next-Generation Clinical Trials for Advanced Therapy Medicinal Products: Strategies, Challenges, and Regulatory Considerations
This comprehensive review examines the evolving landscape of clinical trial design for Advanced Therapy Medicinal Products (ATMPs), including cell and gene therapies.
GMP for Scalable Stem Cell Biomanufacturing: A Guide to Compliance, Automation, and Clinical Translation
This article provides a comprehensive guide for researchers, scientists, and drug development professionals on implementing Good Manufacturing Practice (GMP) for scalable stem cell biomanufacturing.
Navigating EU Cell Therapy Manufacturing License Requirements: A Comprehensive Guide for Researchers and Developers
This article provides a detailed guide for researchers, scientists, and drug development professionals on the regulatory pathway for manufacturing cell therapies in the European Union.
Optimizing iPSC-Cardiomyocyte Differentiation: Protocols, Challenges, and Clinical Applications
This article provides a comprehensive analysis of current protocols for differentiating human induced pluripotent stem cells (iPSCs) into cardiomyocytes (iPSC-CMs).
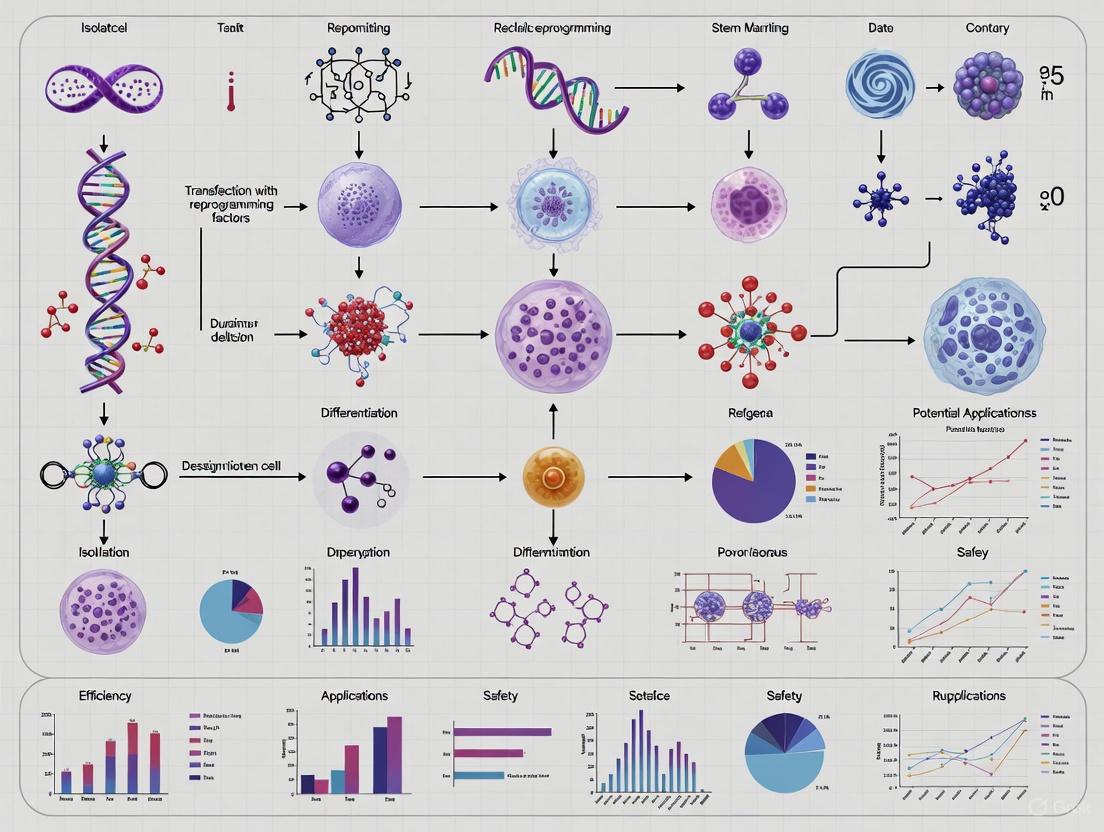
A Comprehensive Protocol for Autologous Induced Pluripotent Stem Cell Therapy: From Reprogramming to Clinical Translation
This article provides a detailed, state-of-the-art overview of the autologous induced pluripotent stem cell (iPSC) therapy pipeline, tailored for researchers, scientists, and drug development professionals.
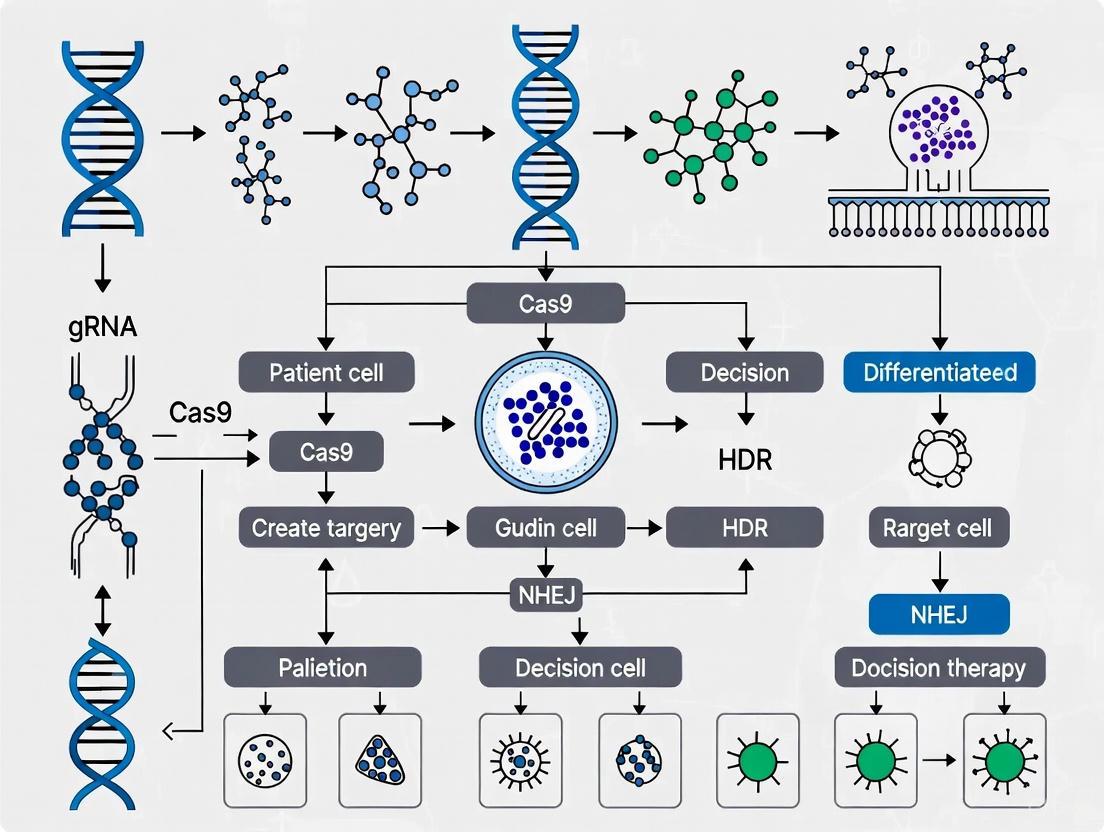
CRISPR-Cas9 and iPSCs: Precision Gene Correction for Therapeutic Development
This article explores the integrated application of CRISPR-Cas9 gene editing and induced pluripotent stem cell (iPSC) technologies for disease correction and therapeutic development.
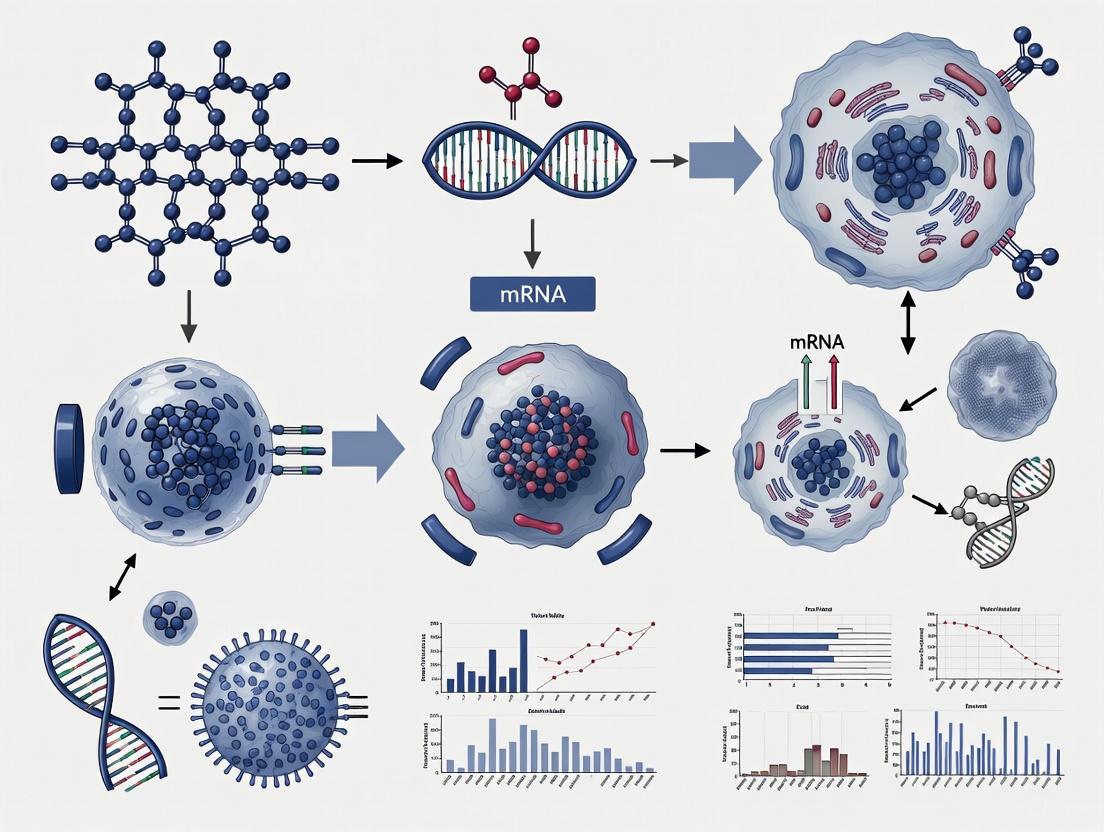
mRNA Reprogramming: A Footprint-Free Path to Clinical-Grade iPSCs and Regenerative Therapies
This article provides a comprehensive overview of mRNA-based technology for cell fate reprogramming, a transformative approach in regenerative medicine and drug development.
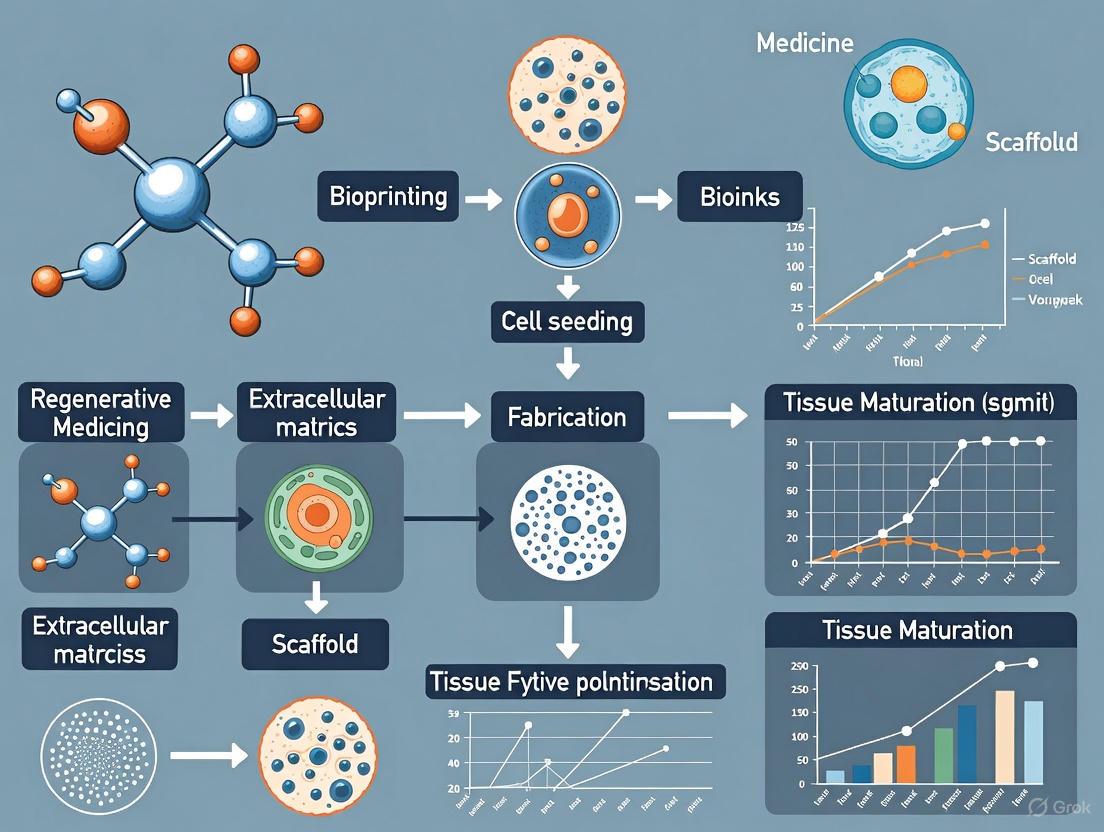
Engineering Complexity: How 3D Bioprinting is Building the Future of Tissues and Therapeutics
This article explores the transformative role of 3D bioprinting in fabricating complex, biomimetic tissue architectures for advanced biomedical applications.
Paracrine Signaling in Stem Cell Therapies: Mechanisms, Applications, and Clinical Translation
This article provides a comprehensive analysis of the paracrine hypothesis in stem cell-based regenerative medicine.
Integrative and Regenerative Pharmacology: Principles, Applications, and the Path to Curative Therapies
This article provides a comprehensive overview of Integrative and Regenerative Pharmacology (IRP), an emerging interdisciplinary field that merges pharmacological sciences, systems biology, and regenerative medicine to develop transformative, curative therapies.